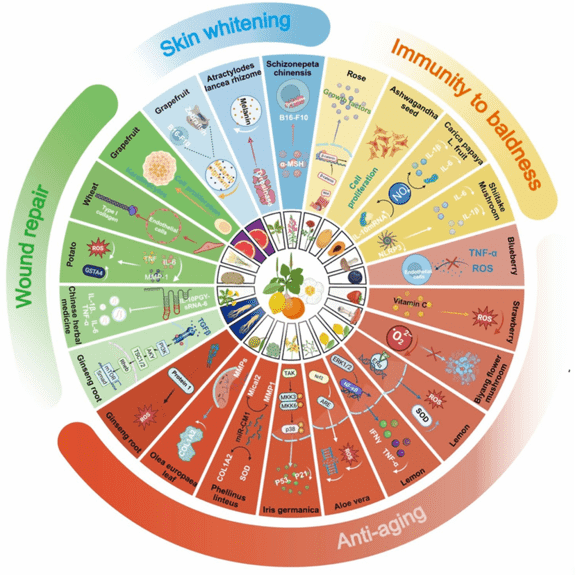
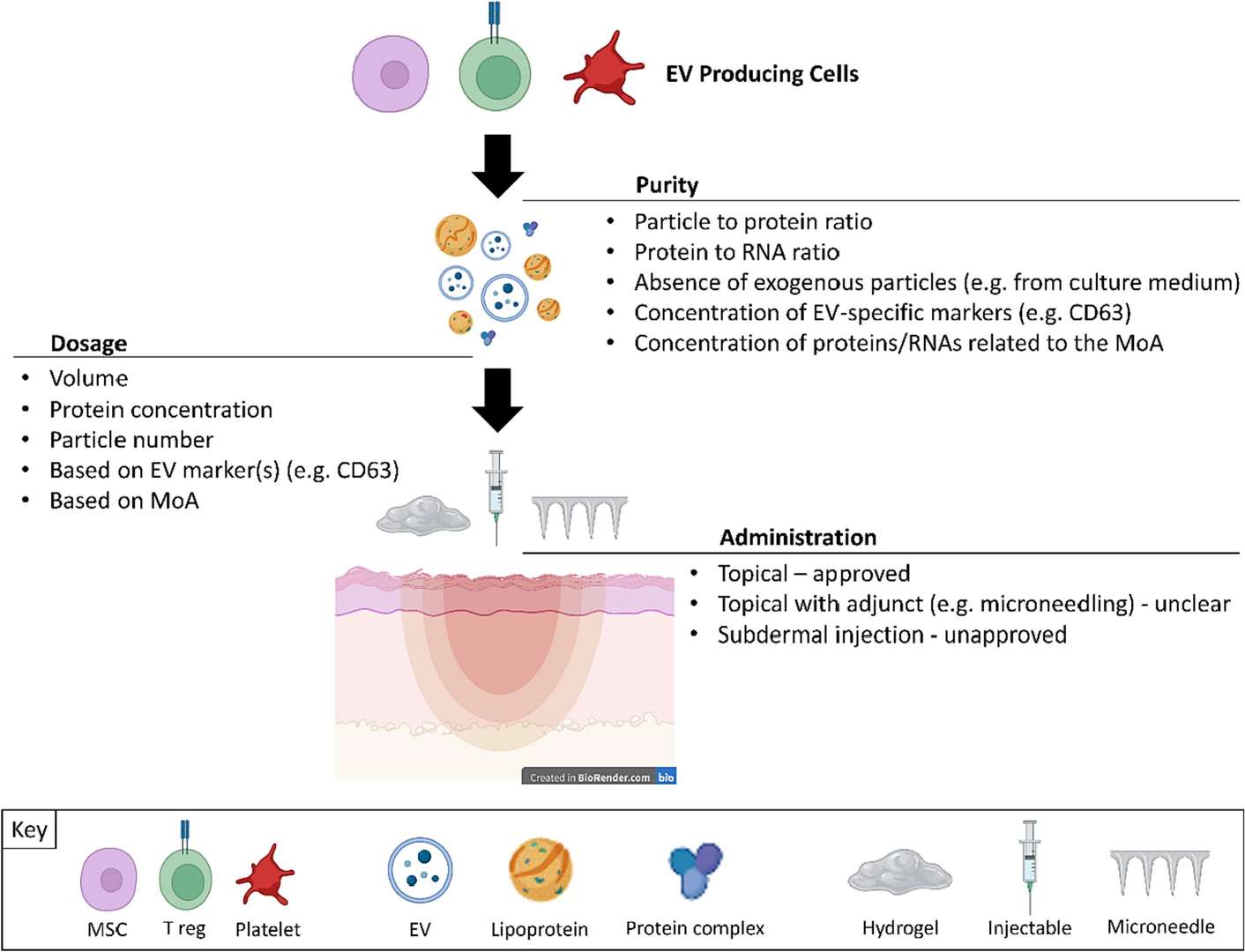
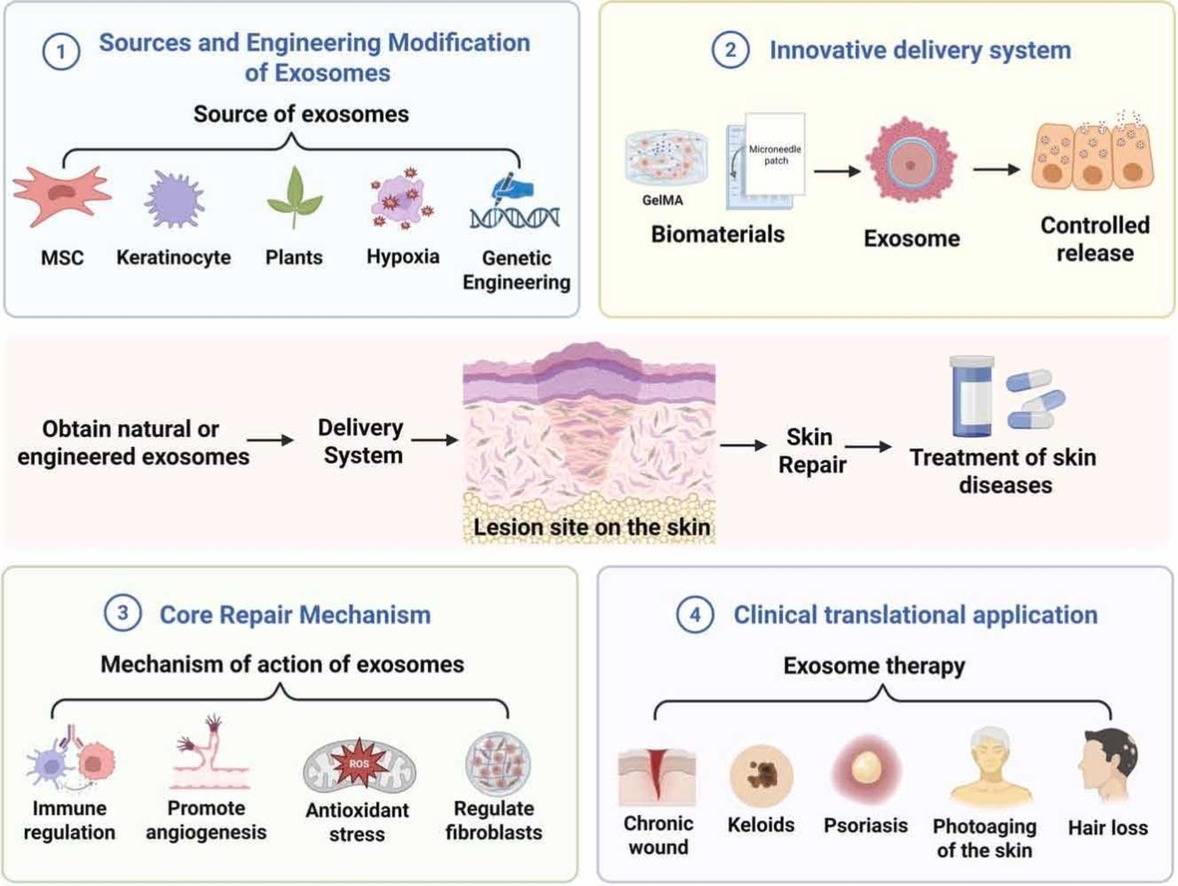
Cosmetic-Grade Stem Cell Exosome Development
The skincare industry is moving beyond traditional chemical actives toward biological regeneration. Mesenchymal Stem Cell (MSC) exosomes are at the forefront of this revolution, offering the regenerative power of stem cells without the regulatory and safety complexities of live cell therapy. However, sourcing consistent, safe, and highly active exosome raw materials remains a major bottleneck for brands.
We provide specialized Cosmetic-Grade Stem Cell Exosome Development solutions. Whether you require exosomes derived from Human Umbilical Cord (hUC-MSCs) for maximum regeneration or Adipose Tissue (hAD-MSCs) for collagen stimulation, our platform delivers high-purity, scalable, and bio-verified raw materials tailored for next-generation anti-aging serums, masks, and post-procedure care products.
Why MSC Exosomes are the Ultimate Skincare Ingredient
Stem cell exosomes act as biological messengers that "wake up" aging skin cells. They offer distinct advantages over plant extracts or peptides.
- Collagen & Elastin Stimulation: MSC exosomes carry specific miRNAs and growth factors that signal dermal fibroblasts to synthesize new Type I Collagen and Elastin, directly reversing signs of aging.
- Rapid Repair: They dramatically accelerate wound healing and reduce inflammation, making them ideal for "Rescue" products used after laser treatments or microneedling.
- Deep Penetration: Being lipid-based nanovesicles (30-150 nm), exosomes penetrate the stratum corneum more effectively than large protein molecules, delivering active cargo deep into the dermis.
- Safety & Stability: Unlike live cells, our cosmetic-grade exosomes are cell-free, sterile, and processed to remove cellular debris, ensuring low immunogenicity and high stability in formulations.
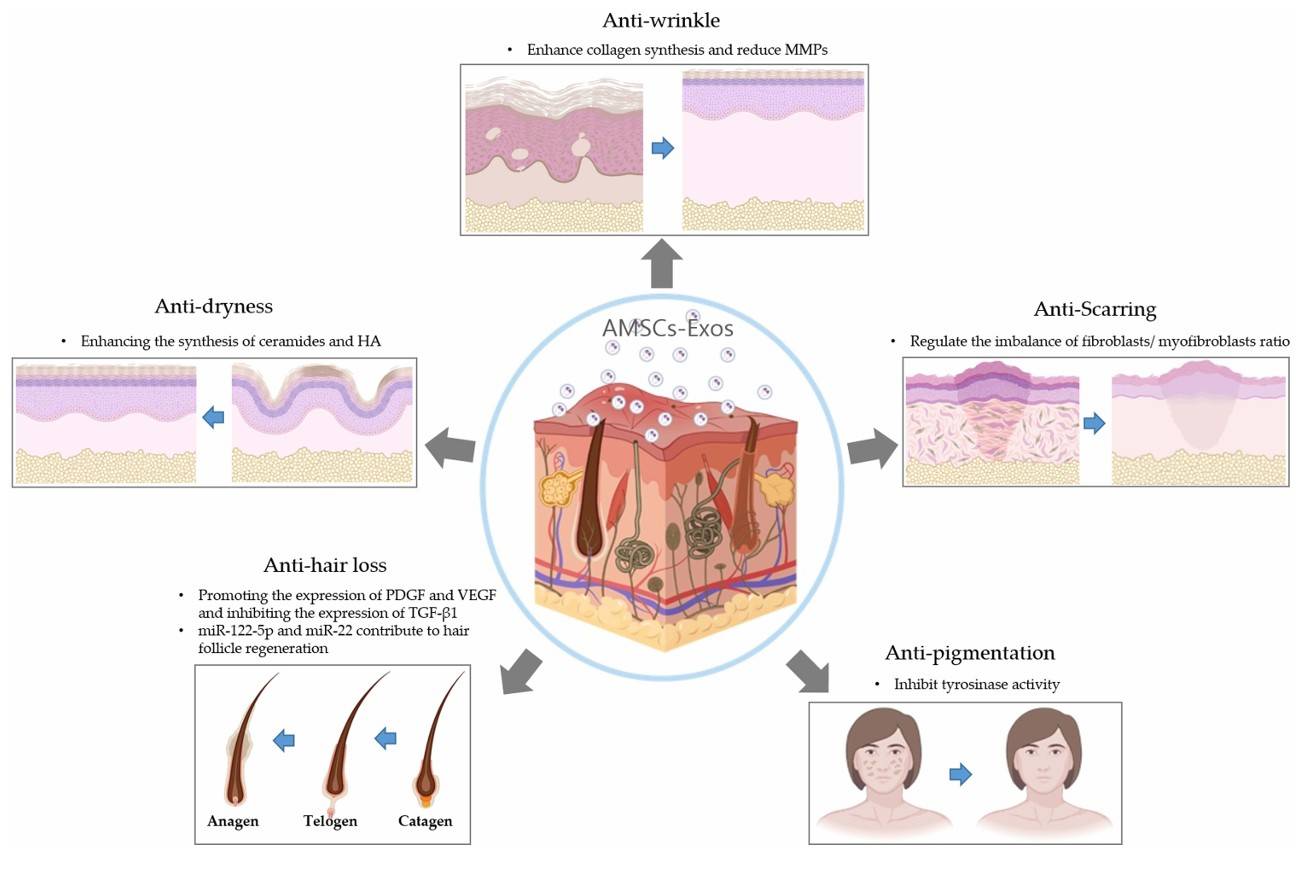 Figure 1. The role of adipose mesenchymal stem cell-derived exosomes (AMSCs-Exos) in skincare during the skin aging process. (Jin YX, et al., 2025)
Figure 1. The role of adipose mesenchymal stem cell-derived exosomes (AMSCs-Exos) in skincare during the skin aging process. (Jin YX, et al., 2025)
Our Cosmetic Raw Material Development Workflow
We offer a "Cell-to-Ingredient" pipeline designed to meet the rigorous safety and efficacy standards of the cosmetic industry.
| Development Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Donor Screening & Cell Banking | Ethical Sourcing: We establish Master Cell Banks from ethically sourced, pathogen-free donors (Umbilical Cord or Adipose). We perform rigorous viral testing to ensure the starting material is safe for cosmetic application. | Animal Tissue Exosome Isolation |
| Scalable Bioproduction | High-Yield Culture: We utilize 3D bioreactor systems to culture MSCs. This scalable approach produces consistent, large-volume batches of conditioned media rich in exosomes, avoiding the variability of flask-based culture. | Upstream Process Development (Cell Culture Optimization) |
| Purification & Safety | Cosmetic-Grade Purity: We employ Tangential Flow Filtration (TFF) to concentrate exosomes while removing media proteins and cellular waste. We rigorously test for endotoxins and mycoplasma to ensure the final ingredient is safe for topical use. | Exosome Purity Analysis, Exosome Isolation by Tangential Flow Filtration (TFF) |
| Efficacy Verification | Bioactivity Claims: We don't just sell particles. We validate every batch using functional assays, such as fibroblast proliferation, scratch wound healing, and collagen synthesis quantification, providing the data needed for your marketing claims. | In Vitro Exosome Functional Assays |
Core Technologies for High-Performance Ingredients
We utilize advanced bio-processing techniques to solve the three biggest challenges in commercializing exosomes: Potency, Purity, and Stability.
3D Bioreactor Scalable Manufacturing
Maximizing Potency & Yield: Traditional 2D flask cultures often yield inconsistent exosomes. We utilize 3D hollow-fiber bioreactors that mimic the in vivo niche. This method not only increases exosome yield by 10-20 fold but also enhances the loading of regenerative cargoes (such as VEGF and bFGF) compared to 2D cultures. This ensures you receive a high-potency ingredient capable of supporting mass-market production.
TFF-Based Purity & Safety Optimization
Removing Contaminants: Safety is paramount in cosmetics. Simple precipitation kits leave behind cytotoxic chemicals and cellular debris. We employ industrial-grade Tangential Flow Filtration (TFF) coupled with chromatography. This process efficiently removes 99% of free proteins (like albumin) and significantly lowers endotoxin levels (LPS), ensuring your product is non-irritating and safe for use on sensitive or compromised skin.
Lyophilization (Freeze-Drying) Formulation
Solving the Stability Crisis: Liquid exosomes degrade rapidly at room temperature, which is unacceptable for consumer products. We have developed proprietary Lyophilization protocols using optimized cryoprotectants (e.g., Trehalose/Mannitol ratios). This technology transforms exosomes into a stable powder that retains bioactivity at room temperature for months and reconstitutes instantly, enabling flexible formulation into vials, masks, or dual-chamber serums.
Application Spotlight: Adipose Exosomes for Skin Barrier Repair and Inflammation Control
This analysis highlights the molecular mechanism by which Adipose MSC exosomes restore compromised skin barriers, providing a high-level scientific basis for sensitive skin formulations.
Featured Technologies:
- Adipose MSC Exosome Isolation
- Efficacy Verification (Anti-Inflammation)
Literature Interpretation:
Skin barrier dysfunction and chronic inflammation are central to conditions like atopic dermatitis and sensitive skin. Researchers investigated the therapeutic potential of small extracellular vesicles derived from adipose-derived mesenchymal stem cells (AD-MSC-sEVs). They found that these sEVs significantly alleviated skin inflammation and restored the epidermal barrier function in disease models. Crucially, the study revealed the mechanism: the sEVs carry bioactive sphingosine kinase 1 (SphK1), which activates the Sphingosine-1-Phosphate (S1P) signaling pathway. This activation upregulates tight junction proteins (like Filaggrin and Claudin-1) and suppresses inflammatory cytokines, effectively reconstructing the skin's physical defense system.
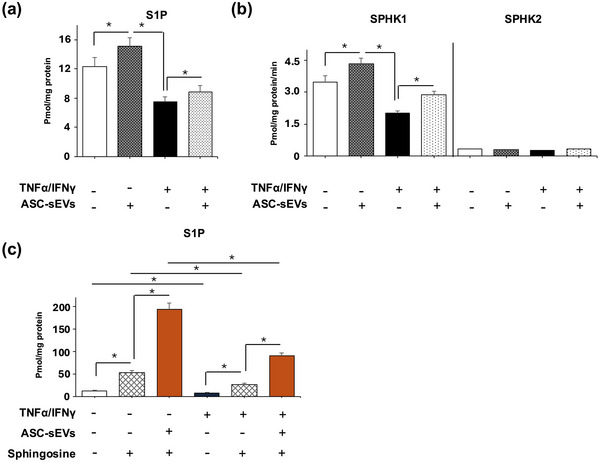 Figure 2. ASC-sEVs enhance SPHK1 activity and S1P production in atopic dermatitis model HaCaT keratinocytes. (a) S1P content, (b) SPHK1 activity, and (c) additional S1P content measurement after treatment with TNF-α, IFN-γ, sphingosine, and ASC-sEVs. (Shin KO, et al., 2025)
Figure 2. ASC-sEVs enhance SPHK1 activity and S1P production in atopic dermatitis model HaCaT keratinocytes. (a) S1P content, (b) SPHK1 activity, and (c) additional S1P content measurement after treatment with TNF-α, IFN-γ, sphingosine, and ASC-sEVs. (Shin KO, et al., 2025)
Start Your Skincare Brand Project
We make getting started straightforward. Our process is designed to be collaborative and transparent.
How It Works: Our Project Pathway
 Figure 3. Our end-to-end workflow for manufacturing safe, high-potency exosome ingredients for the skincare industry. (Creative Biostructure)
Figure 3. Our end-to-end workflow for manufacturing safe, high-potency exosome ingredients for the skincare industry. (Creative Biostructure)
Ready to launch the next "hero ingredient" in beauty? Our scientific team is available for a free consultation to discuss your cosmetic exosome strategy. Contact us today to discuss your project.
References
- Jin YX, Jin GZ. The Anti-Aging Effects of Adipose-Derived Mesenchymal Stem Cell Exosomes on Skin and Their Potential for Personalized Skincare Applications. Clin Cosmet Investig Dermatol. 2025 Sep 12;18:2267-2284.
- Shin KO, Lee JH, Chae S, et al. Small EVs From Adipose-Derived MSCs Modulate Epidermal Barrier and Inflammation Via Sphingosine-1-Phosphate Signaling Pathway. J Extracell Vesicles. 2025 Jul;14(7):e70121.