Viral and Bacterial Infection Exosome Research Services
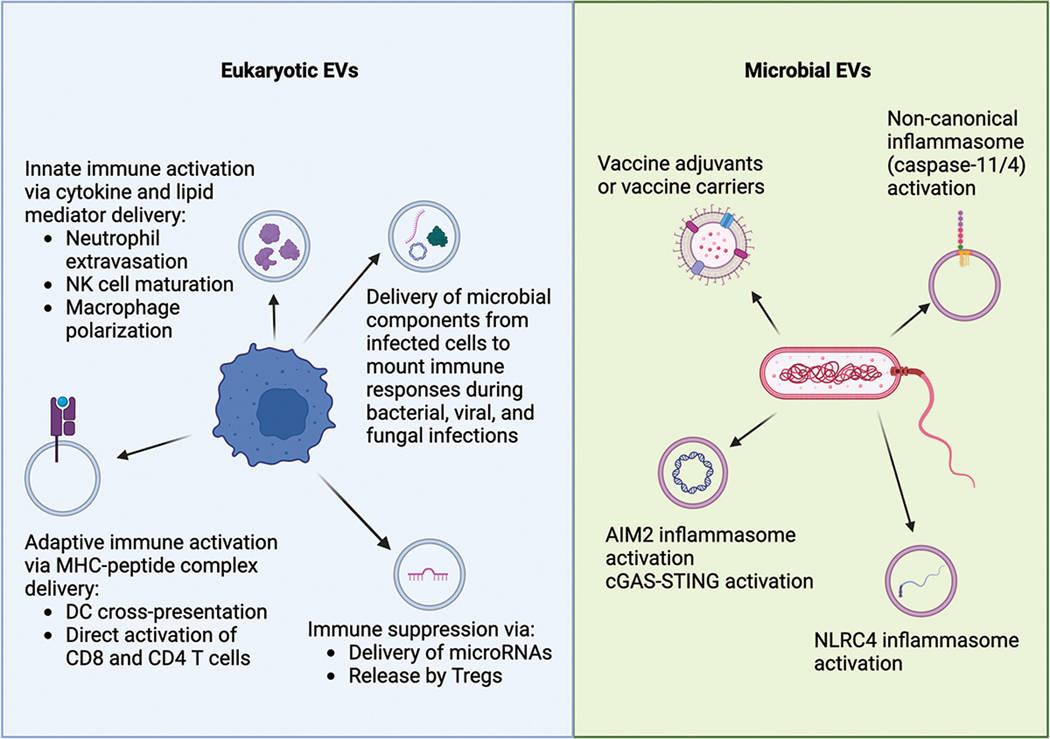
Exosomes and Extracellular Vesicles (EVs) have emerged as critical mediators in the battle between host immune systems and pathogens. In viral infections (e.g., HIV, Hepatitis, SARS-CoV-2), viruses often hijack the host's exosomal machinery to traffic viral RNA or proteins, effectively using exosomes as "Trojan horses" to spread infection or evade immune detection. Conversely, host cells release defensive exosomes acting as "decoys" to neutralize pathogens. In bacterial infections, Outer Membrane Vesicles (OMVs) released by bacteria play key roles in sepsis and virulence.
However, studying these vesicles presents a formidable technical challenge: Co-Isolation. Viruses (typically 80–120 nm) and exosomes (30–150 nm) share nearly identical size and density profiles, making standard ultracentrifugation ineffective for separation. Furthermore, handling infectious samples requires rigorous biosafety protocols. We provide a specialized infectious disease platform designed to distinguish host EVs from pathogenic particles, enabling precise characterization of the "warzone" cargo.
Critical Frontiers in Infectious Disease Research
The study of EVs in infection is unlocking new therapeutic and diagnostic avenues. Key frontiers include:
- The "Trojan Exosome" Hypothesis: Investigating how retroviruses and enveloped viruses exploit the exosomal biogenesis pathway (ESCRT machinery) to bud from cells, making them structurally indistinguishable from host exosomes and shielding them from neutralizing antibodies.
- Bacterial OMVs vs. Host EVs: Deciphering the distinct roles of Bacterial Outer Membrane Vesicles (OMVs) in triggering systemic inflammation (cytokine storms) versus host-derived EVs that may attempt to dampen this response during sepsis.
- Decoy Mechanism & Therapeutics: Exploring how host exosomes carrying receptors (e.g., ACE2 for SARS-CoV-2) bind to pathogens to prevent cellular entry, offering a blueprint for bio-inspired antiviral therapeutics.
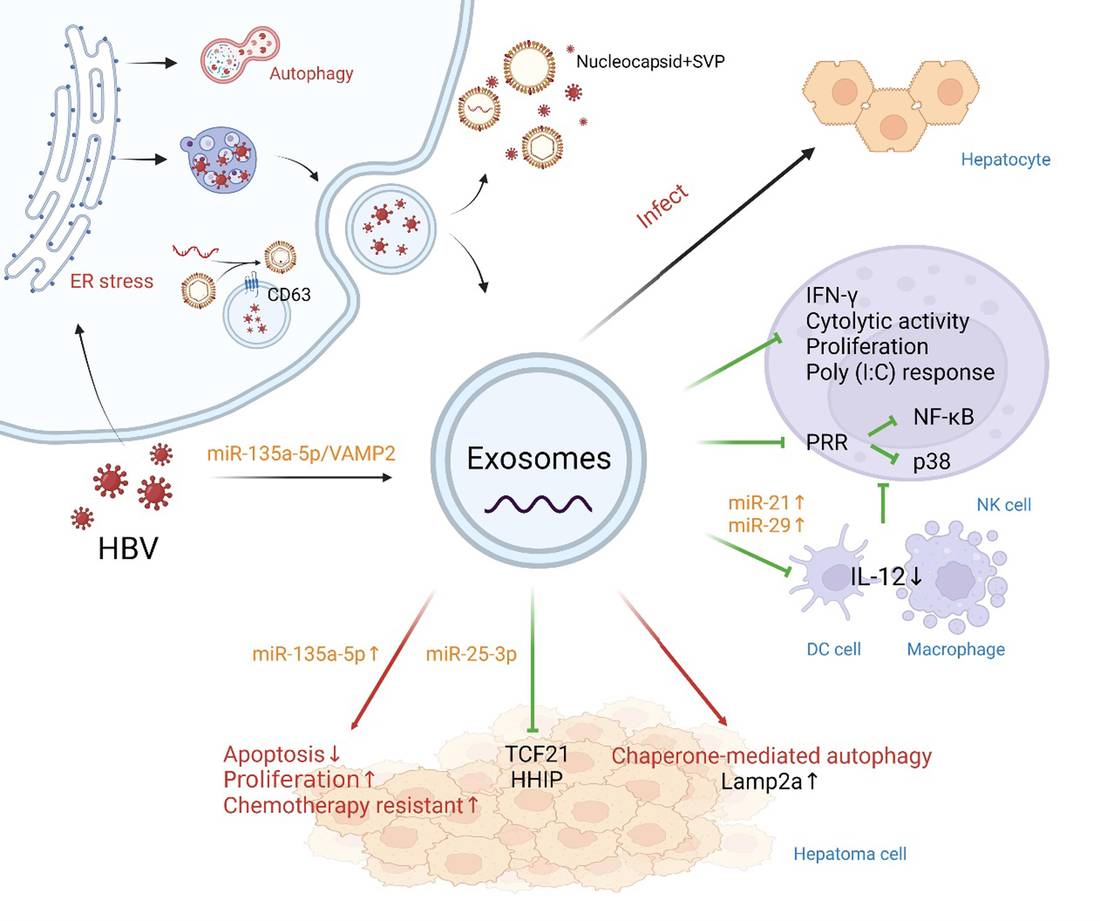 Figure 1. Hepatitis B virus (HBV) propagation via exosomes and its role in disease progression. (Liu Z, et al., 2022)
Figure 1. Hepatitis B virus (HBV) propagation via exosomes and its role in disease progression. (Liu Z, et al., 2022)
Accelerating Pathogen Discovery with Targeted Solutions
We provide a comprehensive portfolio tailored to the biosafety and specificity needs of microbiology and virology research.
| Research Focus | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Distinguishing Virions from Host Exosomes | Immuno-Separation Strategy: Since size-based methods fail, we use Immunoaffinity Capture to pull down CD63/CD81+ host exosomes while leaving viral particles in the supernatant (or vice versa targeting viral envelopes). | Exosome Characterization by NanoFCM, Exosome Isolation by Immunoaffinity Capture |
| Bacterial OMV & Sepsis Profiling | Bacterial EV Isolation: We offer specialized protocols for isolating Outer Membrane Vesicles (OMVs) from bacterial culture supernatants or complex biofluids (plasma/sputum), separating them from host immune vesicles. | Bacterial Extracellular Vesicle (BEV) Isolation, Exosome Quantitative Proteomics |
| Host Immune Response Analysis | Inflammatory Cargo Profiling: We profile the "Cytokine Storm" cargo within exosomes. Using Multi-Omics, we quantify inflammatory miRNAs and cytokines (IL-6, TNF-α) carried by EVs that modulate immune cell behavior. | Exosome Cellular Functional Assays, Immune Response Modulation via Exosomes |
| Exosome-Based Vaccine Development | Pathogen-Mimetic Engineering: We engineer exosomes to display viral antigens (e.g., Spike protein) or use purified bacterial OMVs as natural adjuvants to elicit potent neutralizing antibody responses. | Exosome-Based Vaccine Development, Surface Display Engineering |
Featured Technologies for Infectious Disease
Precise Separation: Virions vs. Vesicles (Immuno-Affinity & SEC)
The greatest hurdle in virology is that enveloped viruses and exosomes are biophysically similar. We employ a combinatorial approach. First, Size Exclusion Chromatography (SEC) removes free viral proteins and soluble cytokines. Second, we utilize Immuno-Affinity Capture targeting tetraspanins (CD63, CD9) to specifically enrich host exosomes, or antibodies against specific viral envelope proteins (e.g., Spike, gp120) to deplete or enrich viral particles. This ensures that the RNA/protein profiles you observe are truly exosomal, not viral contaminants.
High-Resolution Pathogen Visualization (Cryo-EM)
To definitively prove the separation of exosomes from pathogens, we offer Cryo-Electron Microscopy (Cryo-EM). Unlike NTA which only shows particle size, Cryo-EM allows for the visual discrimination of the "spiked" appearance of viruses (like Coronaviruses) versus the smooth lipid bilayer of host exosomes. This morphological validation is often a prerequisite for high-impact publication in infectious disease journals.
BSL-2 Compliant High-Sensitivity Detection (NanoFCM)
Working with infected samples demands safety and sensitivity. Our Nano-Flow Cytometry (NanoFCM) platform is optimized for analyzing small sample volumes from infectious cultures. It can simultaneously detect particle size (down to 40 nm) and surface protein co-expression (e.g., Viral Antigen + Host CD63). This allows researchers to quantify what percentage of circulating exosomes are carrying viral antigens, a key metric for understanding viral pathogenesis and vaccine efficacy.
Application Spotlight: Advanced Models for Chronic HBV-EV Studies
This case study demonstrates the importance of establishing physiologically relevant in vitro models to generate high-quality biofluids for studying host-pathogen interactions over time.
Featured Technologies:
- iPSC-Derived Hepatocyte Differentiation
- Long-Term Infection Supernatant Collection
- Viral Marker Quantification (HBsAg/HBeAg)
Literature Interpretation:
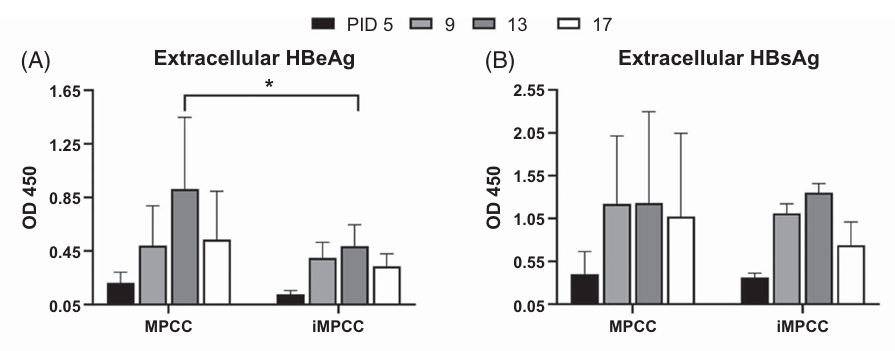 Figure 2. Comparison of HBV infection and hepatic functions between PHH-MPCCs and iMPCCs, with measurements of extracellular HBeAg and HBsAg. (Yuan Y, et al., 2024)
Figure 2. Comparison of HBV infection and hepatic functions between PHH-MPCCs and iMPCCs, with measurements of extracellular HBeAg and HBsAg. (Yuan Y, et al., 2024)
Studying exosome-mediated communication in chronic Hepatitis B Virus (HBV) infection requires stable cell models that mimic the in vivo liver environment. Traditional cell lines often fail to sustain infection. In this study, researchers established a micropatterned co-culture (MPCC) system using iPSC-derived hepatocytes that supported robust HBV infection for over 4 weeks. This breakthrough provides a stable platform for the continuous collection of culture supernatants containing both viral particles and host extracellular vesicles. By utilizing such advanced models, researchers can reliably harvest and separate host exosomes from virions at different stages of chronic infection to decode long-term immune evasion mechanisms.
Start Your Infectious Sample Analysis
We follow strict biosafety and quality control procedures to handle infectious samples, ensuring both safety and data integrity.
How It Works: Our Project Pathway
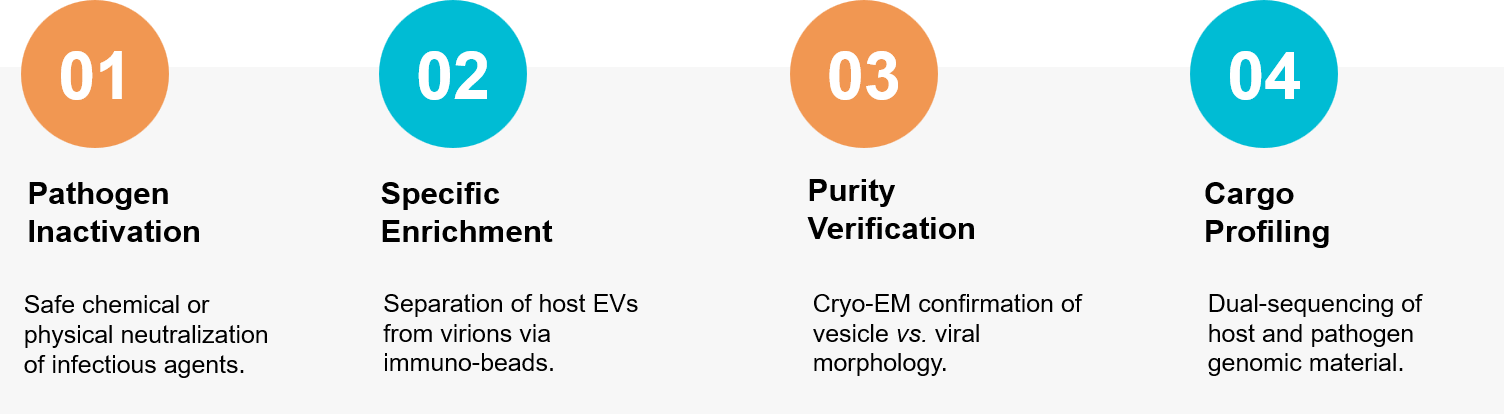 Figure 3. Workflow for separating host exosomes from infectious virions using immuno-affinity and inactivation protocols. (Creative Biostructure)
Figure 3. Workflow for separating host exosomes from infectious virions using immuno-affinity and inactivation protocols. (Creative Biostructure)
Ready to find the next breakthrough in Viral Pathogenesis or Bacterial Sepsis? Our microbiology experts are available to build a custom study plan tailored to your needs. Contact us today to discuss your project.
References
- Liu Z, Li Y, Wang Y, et al. Exosomes in HBV infection. Clin Chim Acta. 2023 Jan 1;538:65-69.
- Yuan Y, Bodke VV, Lin C, et al. Long-term HBV infection of engineered cultures of induced pluripotent stem cell-derived hepatocytes. Hepatol Commun. 2024 Jul 31;8(8):e0506.