Exosome Therapeutics Research Solutions
Cell therapy has revolutionized medicine, but it comes with challenges: storage, viability, and safety risks like tumorigenicity. The industry is shifting.
We provide end-to-end exosome therapeutics solutions. We help you leverage the native biological potency of exosomes—derived from MSCs (Mesenchymal Stem Cells), immune cells, or plants—to develop safe, "off-the-shelf" cell-free therapeutics. Whether you are targeting wound healing, immunomodulation, or tissue regeneration, our platform accelerates your transition from research to exosome-based therapeutic development.
The Shift to Exosome-Based Therapeutics
Why are exosomes based therapeutics becoming the preferred alternative to live cell therapy? Exosomes capture the paracrine "magic" of stem cells without the risks associated with administering live cells.
- Enhanced Safety Profile: Unlike stem cells, therapeutic exosomes cannot replicate or differentiate, eliminating the risk of teratoma formation or uncontrolled division.
- Superior Stability & Storage: Exosomes are stable nanovesicles that can be stored, transported, and administered more easily than live cells, solving critical logistics in the exosome therapeutics market.
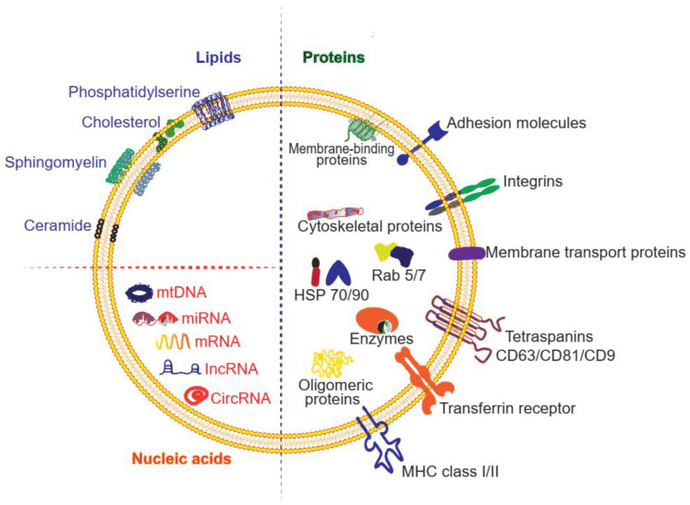
- Low Immunogenicity: MSC exosomes have low expression of MHC antigens, making them ideal candidates for allogeneic (off-the-shelf) therapies with reduced immune rejection risk.
- Biological Barrier Crossing: Their nanoscale size allows them to penetrate tissues that cells cannot reach, including crossing the blood-brain barrier.
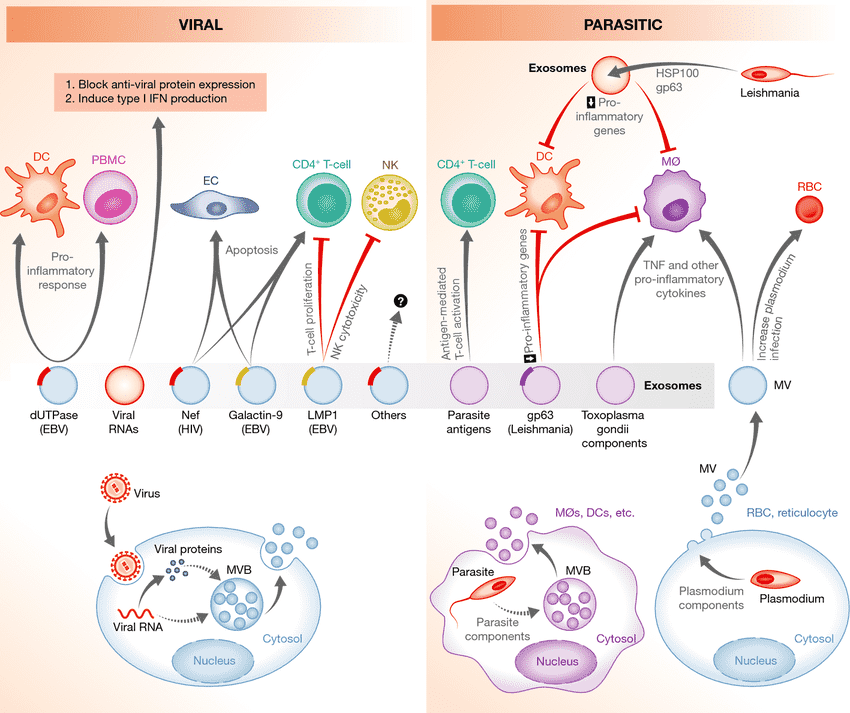
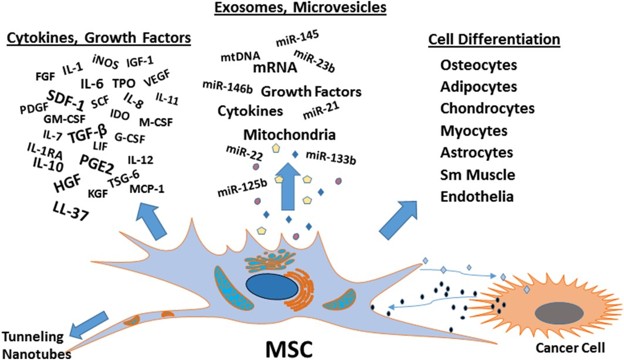 Figure 1. MSC functions and vesicle-based therapy. (Phinney DG, et al., 2017)
Figure 1. MSC functions and vesicle-based therapy. (Phinney DG, et al., 2017)
Our Integrated Therapeutics Development Platform
We offer a seamless workflow for developing exosome-based therapeutics, from cell sourcing to GMP-compliant manufacturing.
| Service Pillar | Key Services & Technologies We Provide |
|---|---|
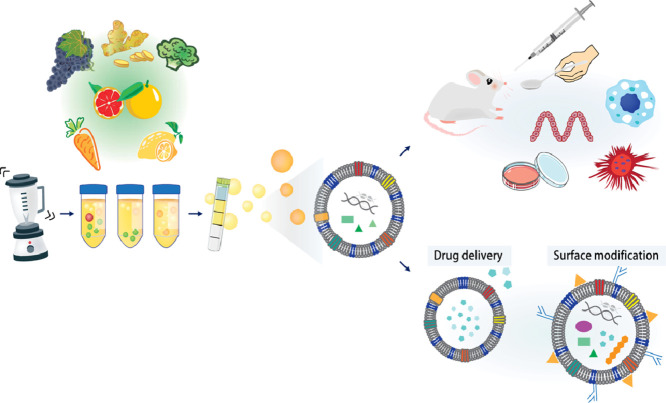
| Cell Source & Isolation | Premium Starting Material: The potency of your therapeutic depends on the cell source. We specialize in isolating exosomes from Human MSCs (Bone Marrow, Umbilical Cord, Adipose), immune cells (DC, Macrophages), and even plant-derived exosome-like nanoparticles. We use scalable TFF to ensure high yield and purity. |
| Potency & Functional Assays | Validating Bioactivity: We don't just count particles; we prove they work. Our functional assays measure the native therapeutic effects, such as angiogenesis (tube formation), anti-inflammatory response, cell migration (scratch assay), and immunomodulation. |
| Engineering for Enhanced Potency | Parental Cell Engineering: To boost therapeutic efficacy, we can engineer the parent cells (e.g., hypoxic preconditioning or cytokine stimulation) to secrete "super-exosomes" enriched with specific regenerative factors. |
| Scalable Manufacturing | From Bench to Clinic: As a specialized partner in exosomes based therapeutics manufacturing, we provide Upstream Process Development (bioreactor expansion) and Downstream Purification to produce clinical-grade batches tailored for exosome therapeutics companies. |
Therapeutic Applications We Support
Our platform supports the research and development of diverse exosome therapeutic models, helping you address unmet medical needs.
MSC and Stem Cell Derived Exosome Therapy
Mesenchymal stem cell (MSC) exosomes are the gold standard for regeneration. We support projects utilizing exosomes from umbilical cord, adipose tissue, and bone marrow to treat ischemic diseases, kidney injury, and osteoarthritis. We help you characterize their cargo (growth factors, miRNAs) and validate their regenerative potential.
Exosome Immunomodulation and Inflammation Control
Exosomes play a key role in regulating the immune system. We assist in developing exosome therapeutics for autoimmune diseases (e.g., RA, IBD) and cytokine storm management. Our assays validate the ability of your exosomes to suppress pro-inflammatory cytokines (TNF-α, IL-6) and promote Treg expansion.
Exosome Wound Healing and Bone Regeneration
Exosomes for skin treatment and orthopedics are a rapidly growing sector. We provide specific models to test exosome efficacy in accelerating wound closure, reducing scarring (exosome therapeutics for scars), and promoting osteogenesis for bone repair applications.
Exosome-Based Vaccine Development
Leveraging dendritic cell-derived exosomes (DEX) as cell-free vaccines. We help profile the antigen-presentation machinery on the exosome surface to develop novel cancer vaccines or anti-viral therapies.
Advantages of Our Therapeutics Platform
Partner with a leader in exosome based therapeutic development.
Comprehensive Potency Assays
Regulatory bodies require proof of potency. We offer a robust suite of in vitro and in vivo assays to quantify the biological activity of your exosomes, linking physical attributes (count/size) to therapeutic outcomes (regeneration/survival).
Scalable Manufacturing Solutions
We solve the bottleneck of manufacturing exosomes a promising therapeutic platform. Our bioreactor-based production and purification workflows ensure you can produce sufficient, consistent material for preclinical and clinical studies.
Multi-Source Expertise
Whether you are investigating amniotic fluid stem cell exosomes or plant-derived exosome-like nanoparticles for their therapeutic activities, our isolation protocols are optimized for diverse biological matrices.
Application Spotlight: Accelerating Wound Healing with MSC Exosomes
This analysis demonstrates the robust regenerative potential of MSC exosomes and highlights the functional assays required to validate them.
Featured Technologies:
- MSC Exosome Isolation
- Functional Assays: Angiogenesis & Migration
Literature Interpretation:
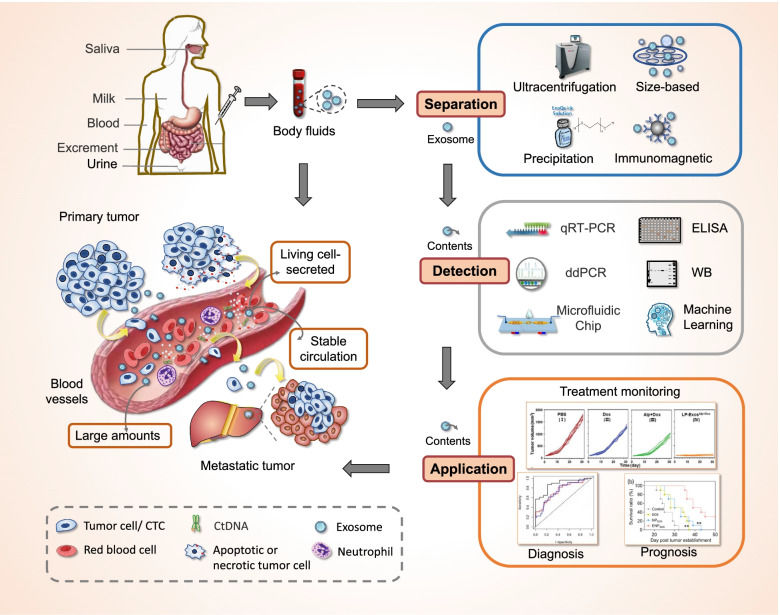
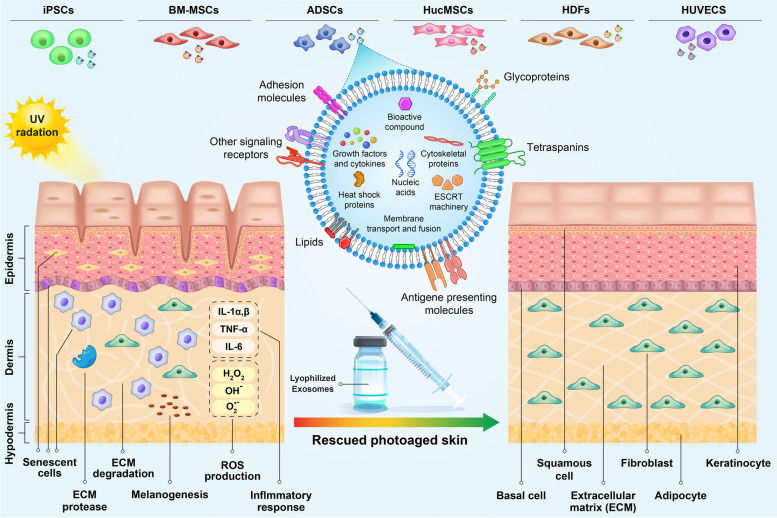
Chronic wounds are a major medical challenge. This study investigated whether exosomes alone (without the stem cells) could accelerate skin repair. Researchers isolated exosomes from mesenchymal stem cells (MSCs) and tested them using standard functional assays. The exosomes successfully induced the proliferation and migration of fibroblasts (verified by scratch assays) and promoted new blood vessel formation (verified by tube formation assays). Crucially, in animal models, the exosome-treated wounds closed significantly faster than controls.
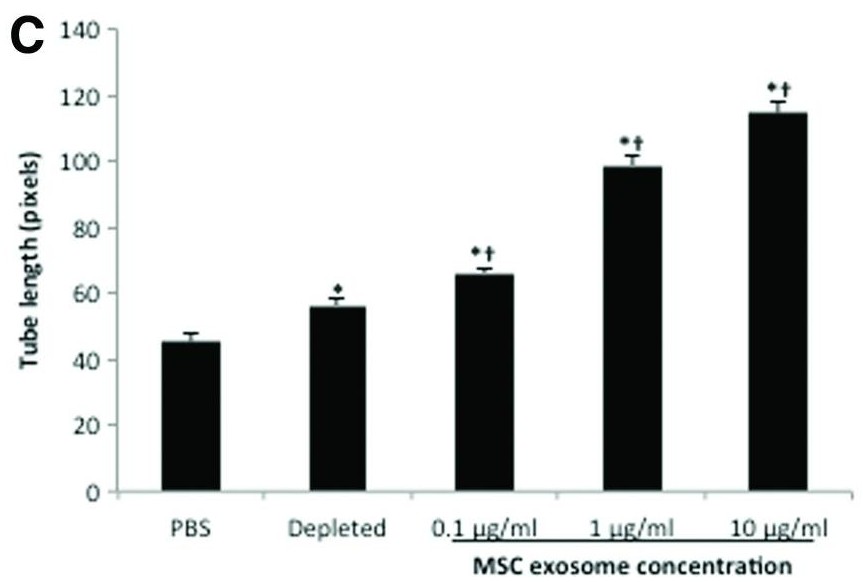 Figure 2. MSC exosomes boost tube formation in vitro. (Shabbir A, et al., 2015)
Figure 2. MSC exosomes boost tube formation in vitro. (Shabbir A, et al., 2015)
Start Your Therapeutic Development Project
We make getting started straightforward. Our process is designed to be collaborative and transparent, ensuring your project goals are met at every stage.
How It Works: Our Project Pathway
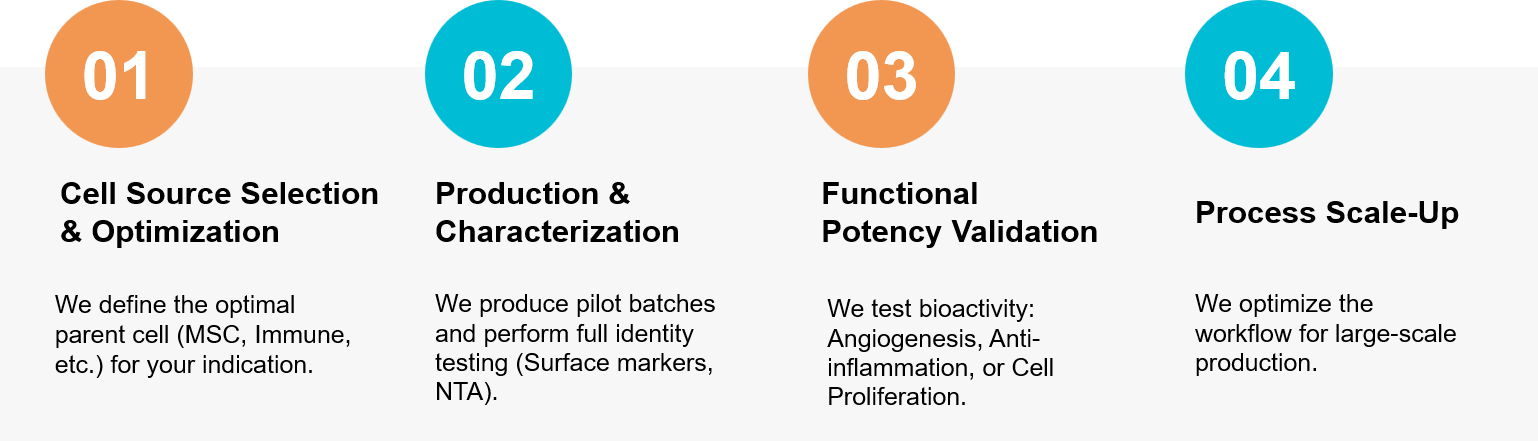 Figure 3. Project Workflow for Cell Source Selection to GMP Manufacturing Process. (Creative Biostructure)
Figure 3. Project Workflow for Cell Source Selection to GMP Manufacturing Process. (Creative Biostructure)
Ready to develop the next generation of medicines? Our scientific team is available for a free consultation to discuss your exosome therapeutic strategy.
References
- Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017 Apr;35(4):851-858.
- Shabbir A, Cox A, Rodriguez-Menocal L, et al. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015 Jul 15;24(14):1635-47.