Biofluid Exosome Isolation Service
Biofluid-derived exosomes offer a powerful window into cellular activity and disease states. These nanoscale vesicles, naturally secreted into fluids such as blood, urine, and cerebrospinal fluid, carry molecular signatures that reflect the physiological conditions of their originating cells.
At Creative Biostructure, we provide specialized exosome isolation services tailored to the complexity of biological fluids. Our protocols are designed to consistently yield high-purity extracellular vesicles suitable for diagnostic discovery, therapeutic research, and advanced molecular profiling. With deep expertise and robust infrastructure, we support your research by enabling reliable access to exosomes even from limited or challenging biofluid samples.
Why Focus on Body Fluid-Derived Exosomes?
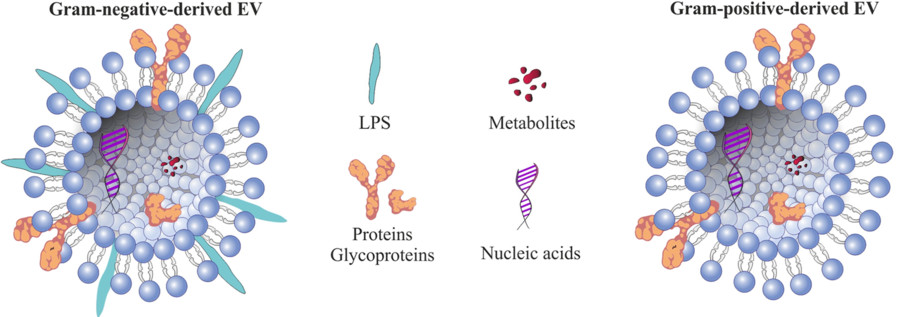
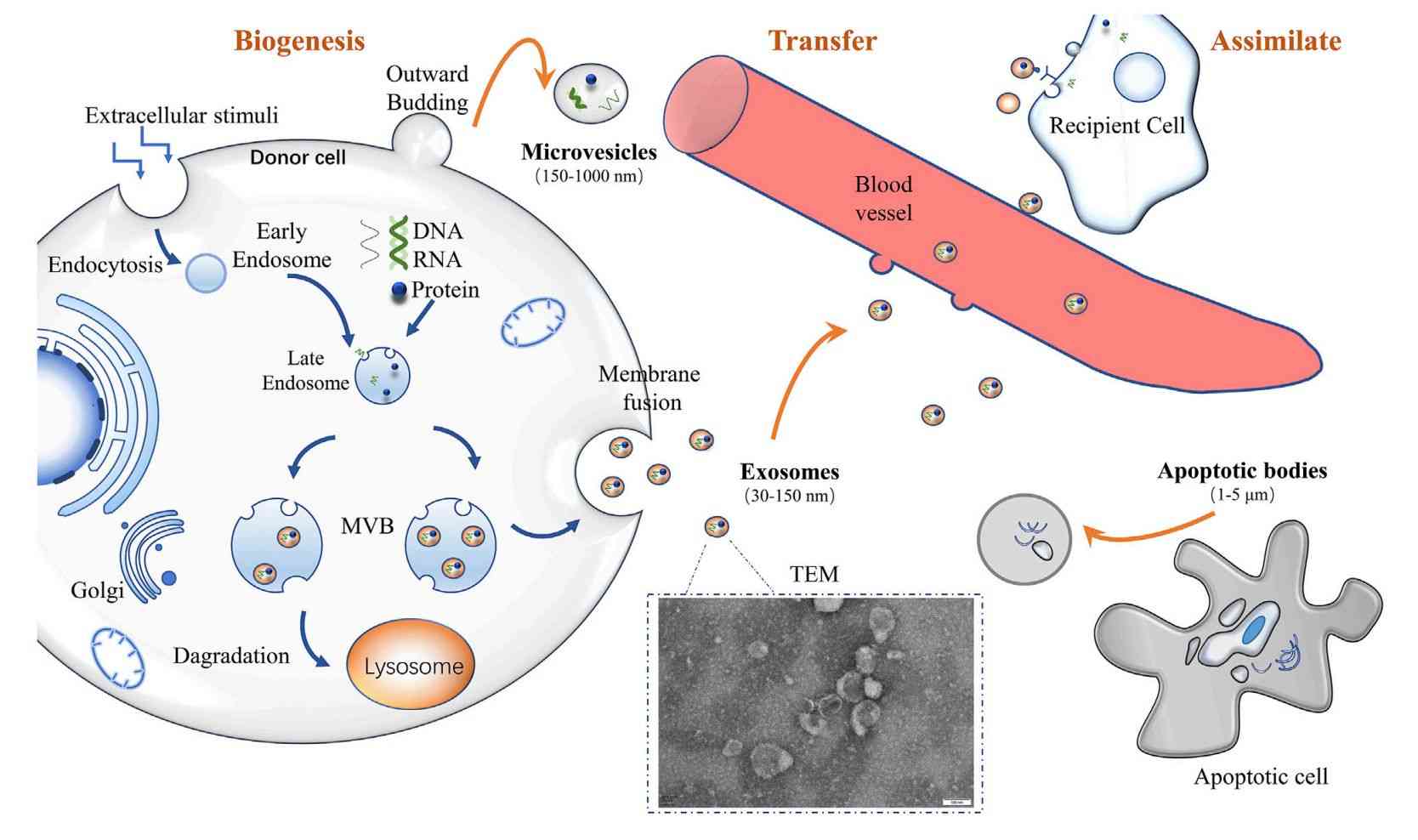
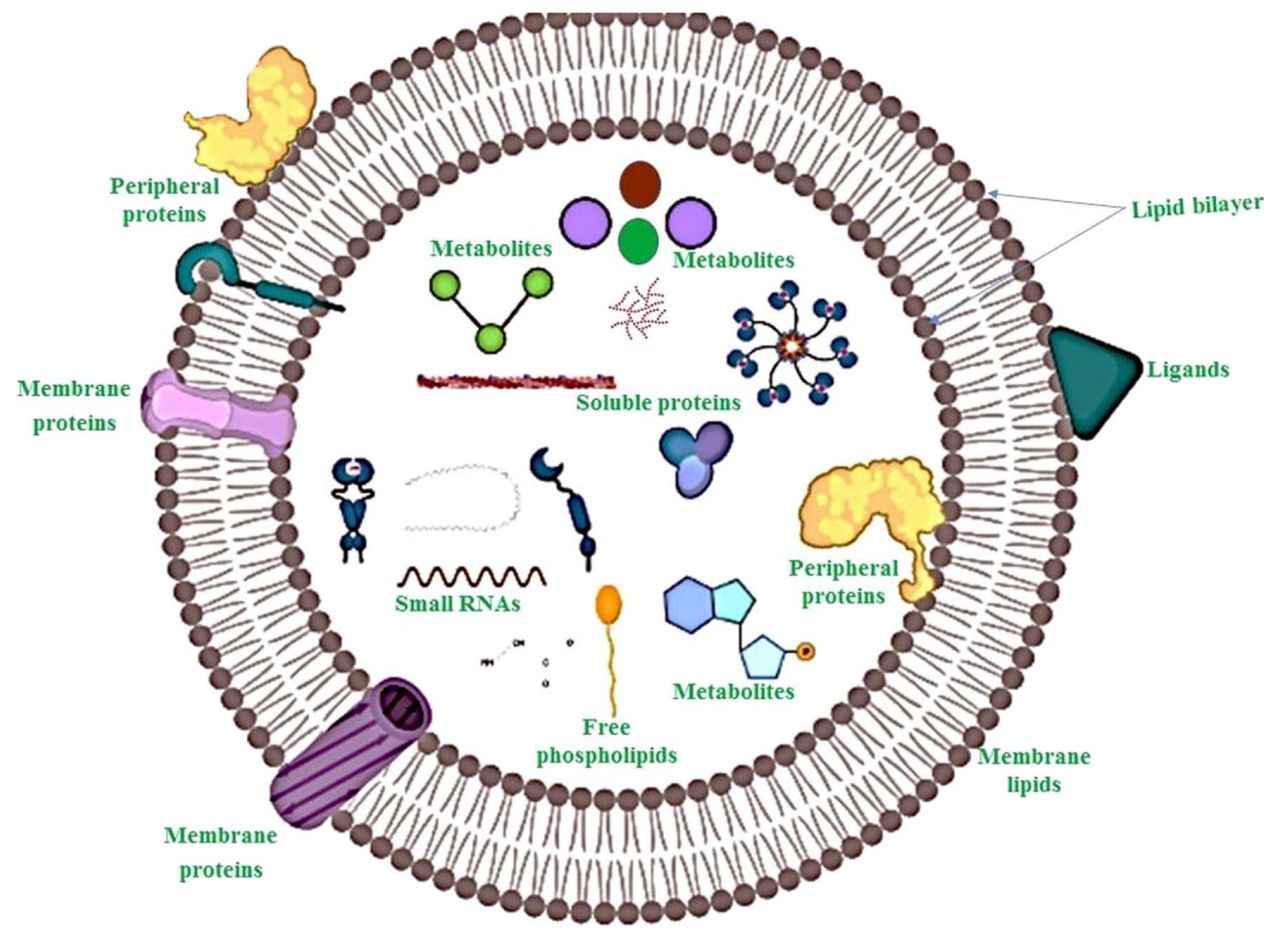
Exosomes are nanoscale extracellular vesicles (EVs), typically measuring between 30 and 160 nanometers in diameter. They are secreted by nearly all cell types and can be found in common biological fluids such as plasma, serum, urine, cerebrospinal fluid (CSF), and saliva. These vesicles carry a diverse range of molecular cargos, including proteins, RNAs, lipids, and metabolites that reflect the physiological or pathological state of their originating cells.
Isolating exosomes from biofluids offers several key advantages:
- Easy access through non-invasive sampling
- Real-time insight into physiological and pathological processes
- Relevance to conditions such as cancer and neurodegenerative diseases
- Compatibility with biomarker discovery and therapeutic delivery
These features make biofluid-derived exosomes valuable tools for liquid biopsy, immune profiling, disease monitoring, and translational research.
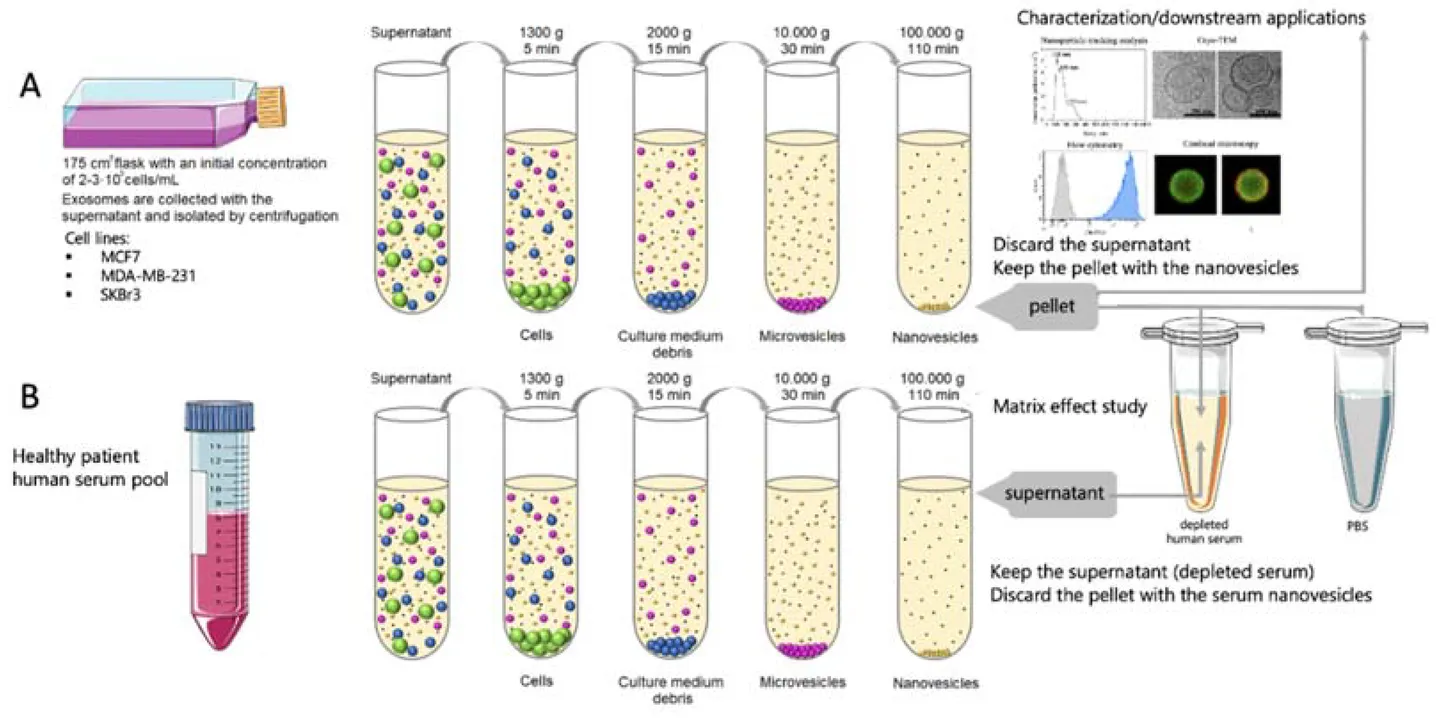
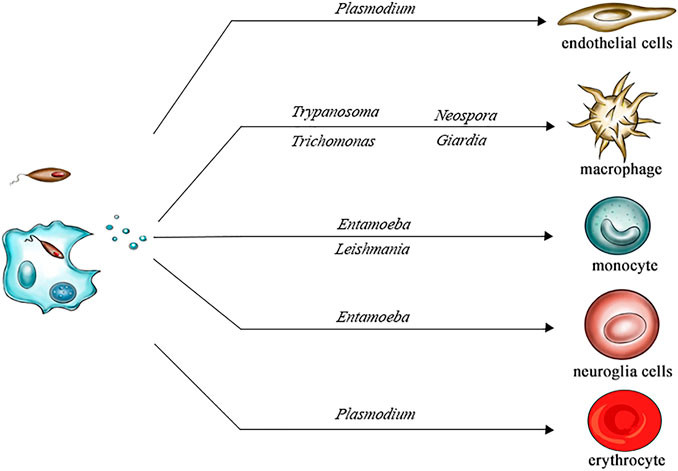
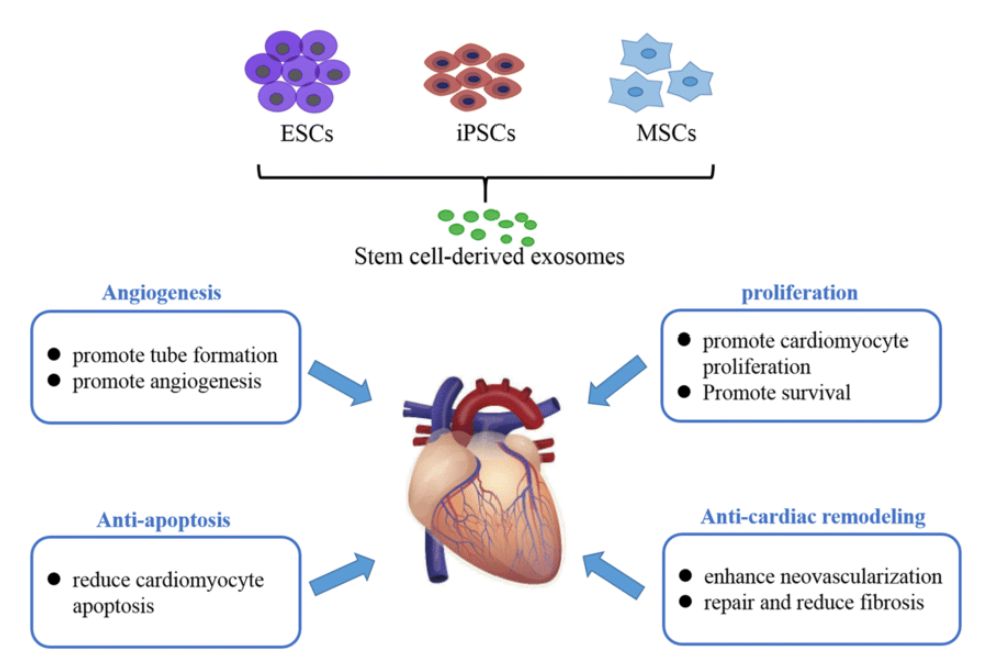
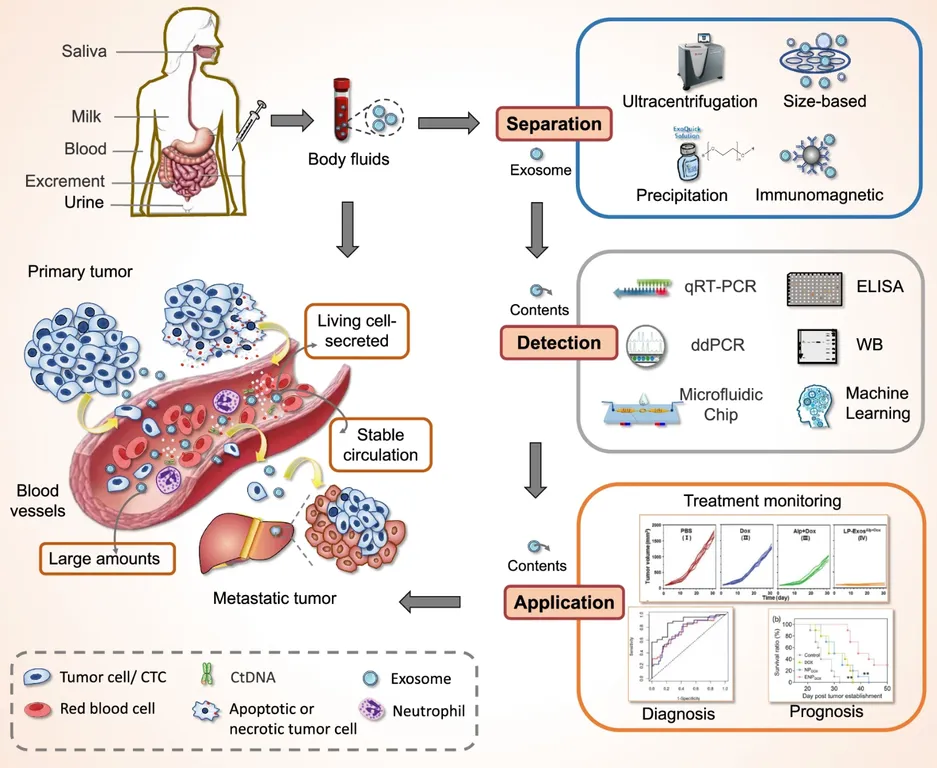 Figure 1. Exosomes Derived from Body Fluids as Emerging Biomarkers for Liquid Biopsy. (Yu D, et al., 2022)
Figure 1. Exosomes Derived from Body Fluids as Emerging Biomarkers for Liquid Biopsy. (Yu D, et al., 2022)
What Biofluids Do We Support?
Our team is experienced in isolating exosomes from a variety of biological fluids, including but not limited to:
- Plasma and Serum: Ideal for systemic biomarker studies and disease progression monitoring. We optimize for high-purity EVs while minimizing platelet- and protein-based contaminants.
- Urine: A low-protein matrix enabling detection of urogenital and systemic disease-related EVs.
- Cerebrospinal Fluid (CSF): Suitable for neuroscience and neurodegeneration studies; our workflow maximizes yield from limited sample volumes.
- Saliva: Non-invasive sampling source rich in EVs relevant to oral and gastrointestinal conditions.
- Breast Milk, Amniotic Fluid, Semen, and Synovial Fluid: Custom protocols available upon request for rare or specialty fluids.
Our Isolation Platforms and Technologies
Creative Biostructure provides a variety of specialized exosome isolation methods designed to suit different types of biofluids, purity levels, and research goals. The table below outlines the key technologies we apply, along with their core features and recommended use cases.
| Technology Name | Description | Recommended Applications |
|---|---|---|
| Polymer-based Precipitation | Uses water-excluding polymers to aggregate exosomes at low-speed centrifugation. Fast, simple, and requires minimal equipment. | High-throughput screening, small sample volumes, pilot studies |
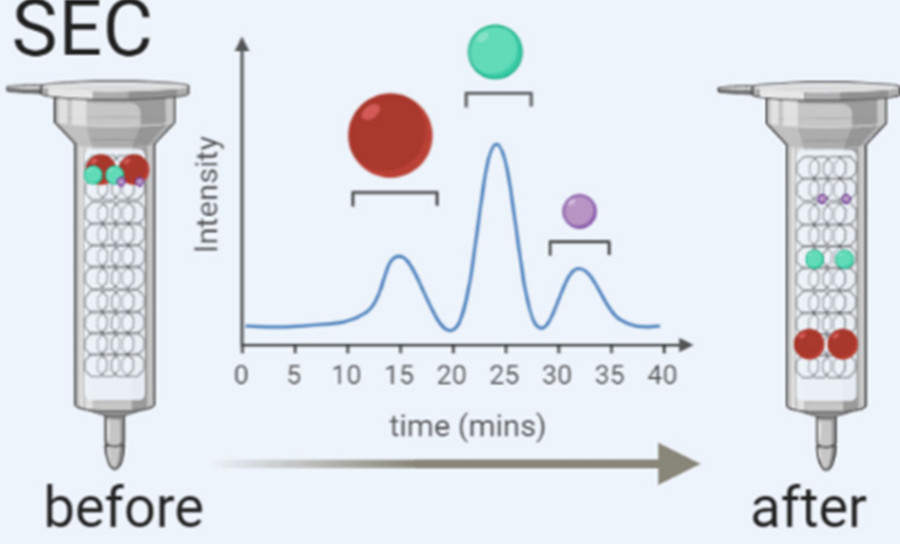
| Size-Exclusion Chromatography (SEC) | Separates exosomes based on size using porous beads. Yields high-purity fractions with minimal protein contamination. | Proteomics, biomarker discovery, downstream functional assays |
| Column-based Kits | Pre-packed columns designed for EV isolation from specific fluids. Often use SEC principles with pre-optimized conditions. | Small-volume clinical samples, translational research |
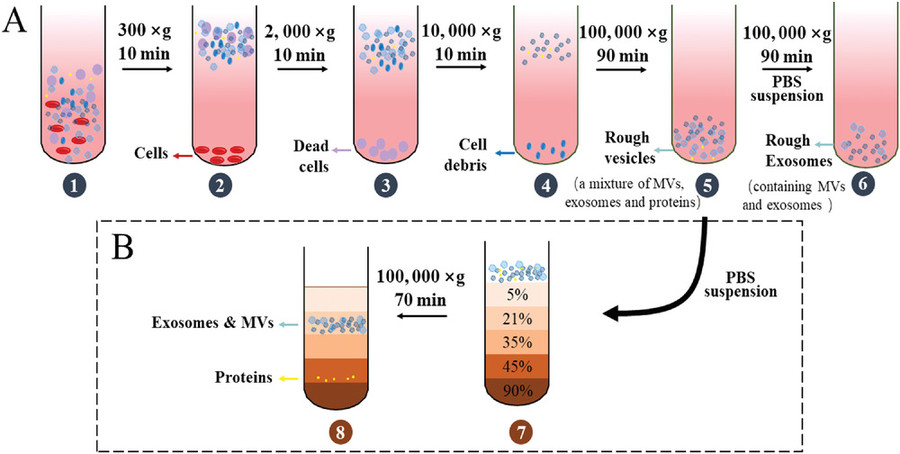
| Differential Ultracentrifugation | Traditional gold-standard method using sequential high-speed spins to pellet exosomes. | Large-volume samples, protocol standardization needs |
| Tangential Flow Filtration (TFF) | Membrane-based filtration technique suitable for volume reduction and concentration of EVs. | GMP-compliant workflows, scalable manufacturing |
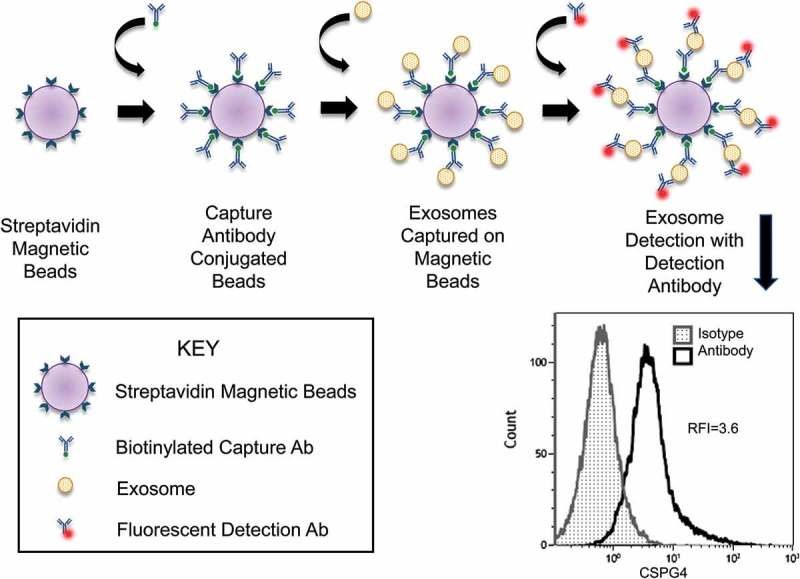
| Immunoaffinity Capture | Selectively isolates exosome subsets using surface marker-specific antibodies. | Subpopulation analysis, diagnostic target enrichment |
In addition, we offer flexible, integrated workflows that combine multiple isolation strategies to maximize exosome recovery, purity, and compatibility with downstream applications.
Our Biofluid Exosome Isolation Workflow
At Creative Biostructure, each biofluid exosome isolation project follows a standardized yet customizable workflow to ensure reproducibility and high-quality results:
Consultation and Method Selection
We begin with a detailed assessment of your sample type, volume, and research goals. Based on this information, our experts recommend the most suitable isolation strategy.
Sample Processing
Upon receipt, all samples are logged, aliquoted, and processed under controlled conditions. Pre-treatment steps such as centrifugation or filtration are applied to remove debris and minimize contamination.
Exosome Isolation
Isolation is carried out using validated protocols tailored to each fluid type. We employ SEC, precipitation, ultracentrifugation, or hybrid approaches depending on the application.
Optional Characterization
Clients may request additional services such as NTA, NanoFCM, TEM, zeta potential, or Western blotting for comprehensive exosome profiling.
Quality Control
Each batch undergoes internal quality checks to assess particle size distribution, yield, and purity. Reports can be included upon request.
Data Delivery and Sample Return
Isolated exosomes are shipped on dry ice in PBS or a custom buffer, accompanied by a project summary and optional characterization data.
This streamlined process ensures that every project delivers consistent, reliable, and publication-ready exosome preparations.
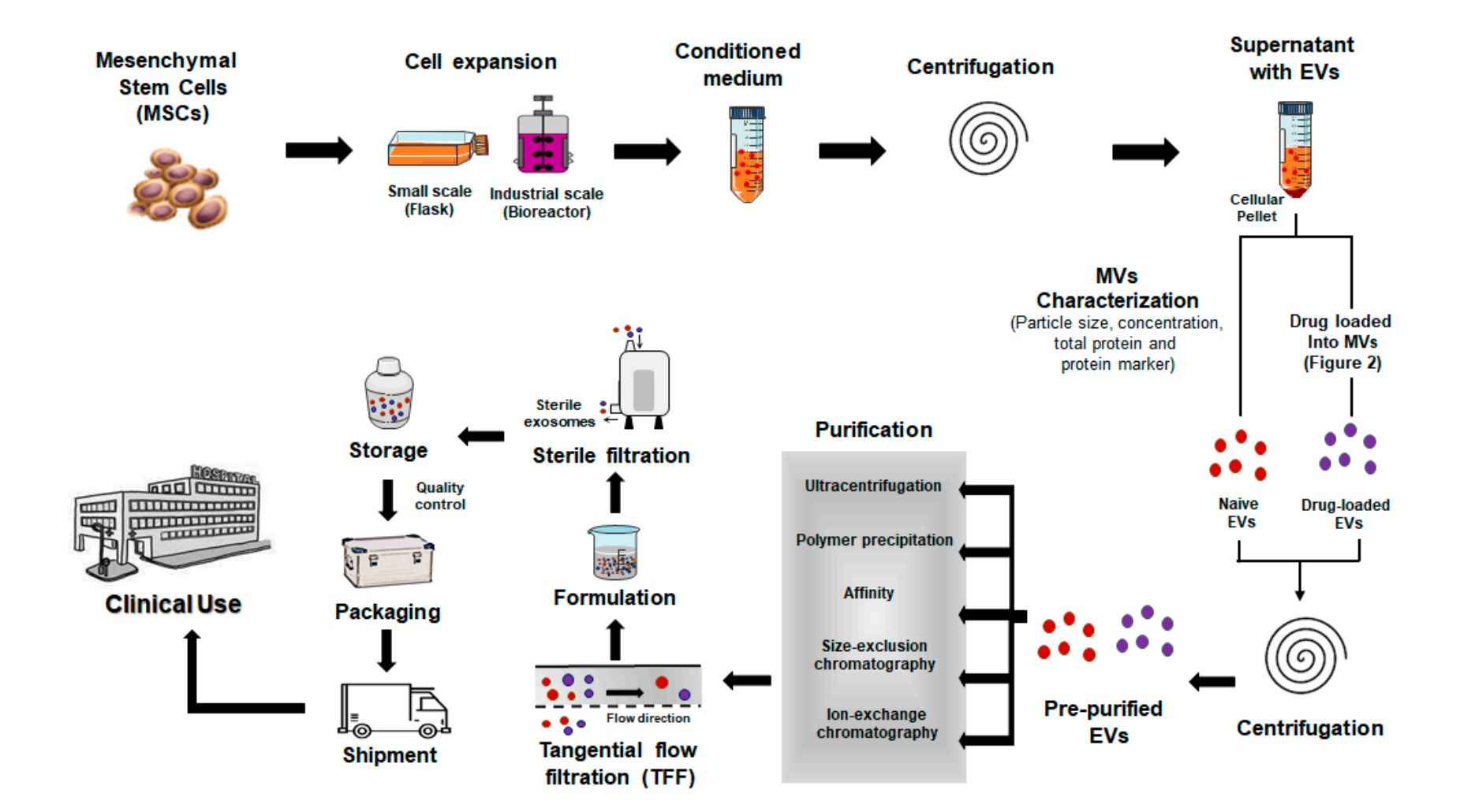
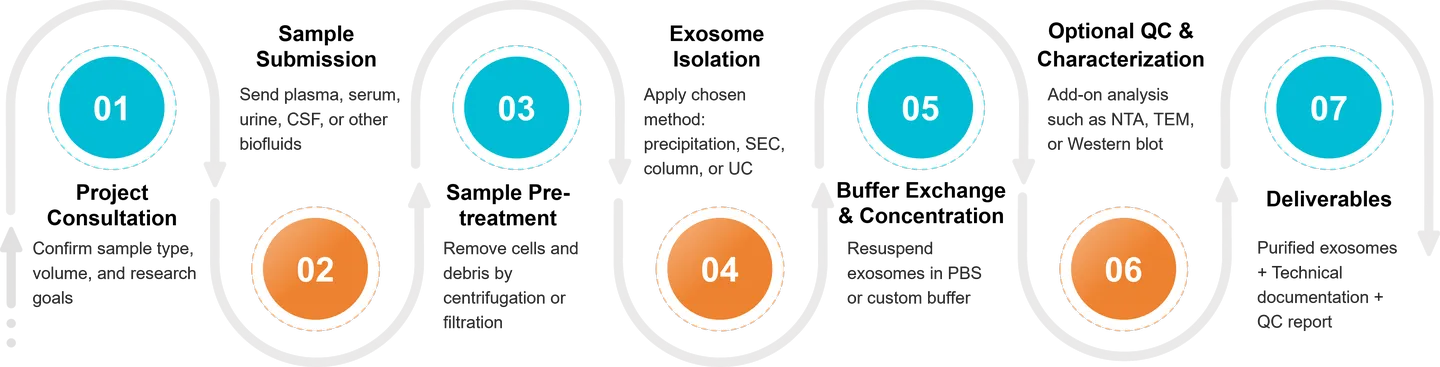 Figure 2. Biofluid Exosome Isolation Project Workflow. (Creative Biostructure)
Figure 2. Biofluid Exosome Isolation Project Workflow. (Creative Biostructure)
Sample Requirements
To ensure optimal isolation results, please prepare and submit samples according to the following guidelines:
- Sample Types Accepted: Plasma, serum, urine, cerebrospinal fluid, saliva, breast milk, amniotic fluid, and other research-grade biofluids.
- Recommended Volume:
- Plasma/Serum: ≥ 250 µL
- Urine: ≥ 1 mL
- CSF: ≥ 3 mL
- Other fluids: Contact us for guidance
- Storage Conditions: Samples should be stored at -80°C prior to shipping. Avoid repeated freeze-thaw cycles.
- Shipping Instructions: Ship samples on dry ice with proper labeling and documentation. Include any relevant details such as anticoagulants or pre-treatment steps.
- Special Considerations: Please inform us if samples may contain infectious agents or require biosafety precautions. Custom protocols are available upon request.
If you have limited sample volume or special handling needs, please contact our technical team for personalized support before submission.
Quality Control and Deliverables
Each project undergoes strict internal quality control to ensure the integrity and consistency of the exosome preparations.
Quality Control Options (available upon request):
- Nanoparticle Tracking Analysis (NTA): Size distribution and particle concentration
- Transmission Electron Microscopy (TEM): Vesicle morphology confirmation
- Western Blotting: Detection of exosomal markers (e.g., CD63, CD81, TSG101)
- Zeta Potential Analysis: Stability evaluation
- Protein and RNA Quantification: Assessment of sample content and purity
Standard Deliverables:
- Purified exosomes in PBS or custom buffer
- Technical summary including method details and yield
- Optional QC report based on selected analysis
- Digital data files (e.g., NTA plots, TEM images, Western blot scans) if applicable
We ensure all deliverables are shipped on dry ice with proper labeling and documentation. Additional services or data formatting can be customized to meet specific research or publication requirements.
Key Benefits of Our Biofluid Exosome Isolation Service
- Small Sample Volume Compatible: As low as 250 µL for plasma or serum
- High Yield and High Purity: Optimized protocols for superior recovery
- Broad Fluid Compatibility: Validated across multiple body fluids
- Flexible Workflow: Select the isolation method best suited to your study
- Downstream Ready: Purified exosomes are suitable for omics, functional, or therapeutic studies
Case Study
Case: UF-SEC-Based Isolation of CSF-Derived Extracellular Vesicles for Proteomic Profiling
Background
Cerebrospinal fluid (CSF)-derived extracellular vesicles (EVs) are promising biomarkers for neurological diseases, but their isolation is challenged by low sample volume and contaminant proteins. This study validated an ultrafiltration combined with size-exclusion chromatography (UF-SEC) approach for isolating EVs from canine CSF.
Key Findings- Optimal Fraction: SEC fraction 3 was enriched with EV markers (e.g., RAB5C, CDC42) and showed minimal contamination by albumin or ApoA1.
- Proteomic Yield: Up to 1493 ± 107 proteins were identified from 6 mL CSF; even 0.5 mL yielded 743 ± 77 proteins with the MBR setting.
- Reproducibility: 180 core proteins were shared across all volumes, including 36 from the Vesiclepedia top 100 list.
- Practical Insight: Sufficient EVs can be isolated from as little as 0.5 mL CSF, enabling analysis in small-volume or clinical samples.
UF-SEC enables high-quality EV isolation from minimal CSF input, supporting reliable downstream proteomic analysis and expanding feasibility for translational CNS biomarker studies.
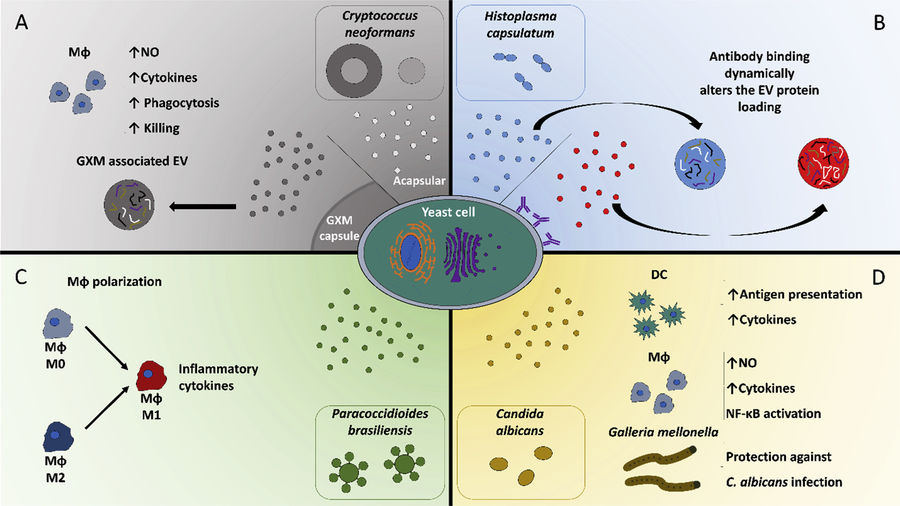
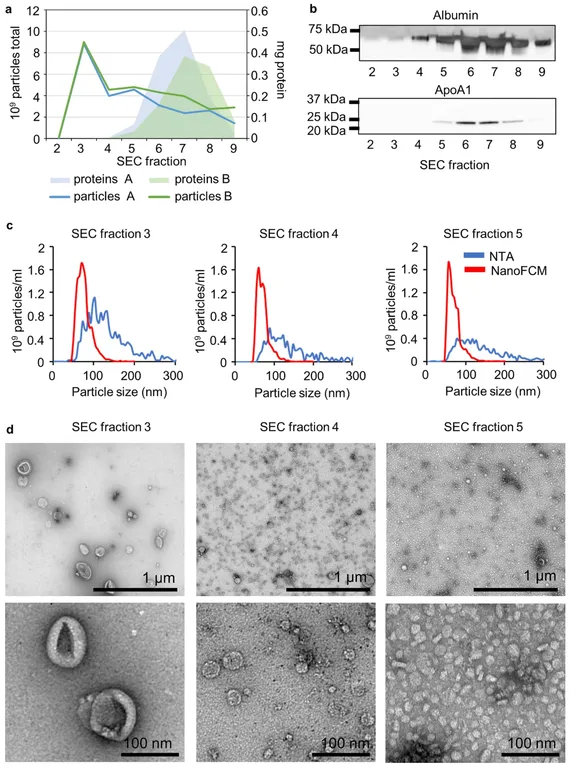 Figure 3. EV Characterization from CSF Using UF-SEC. (a) Particle concentration (NTA) and protein content across SEC fractions 2-9 from two CSF pools. (b) Western blot detection of albumin and ApoA1 in fractions 2-9 from pool A. (c) Particle size profiles analyzed by NTA and NanoFCM from fractions 3-5 of pool B. (d) TEM images showing vesicle morphology in fractions 3-5 from pool B. (Kangas P, et al., 2023)
Figure 3. EV Characterization from CSF Using UF-SEC. (a) Particle concentration (NTA) and protein content across SEC fractions 2-9 from two CSF pools. (b) Western blot detection of albumin and ApoA1 in fractions 2-9 from pool A. (c) Particle size profiles analyzed by NTA and NanoFCM from fractions 3-5 of pool B. (d) TEM images showing vesicle morphology in fractions 3-5 from pool B. (Kangas P, et al., 2023)
Ready to accelerate your exosome research? Creative Biostructure offers customized biofluid exosome isolation services with high yield, purity, and reliability. Contact us to discuss your sample types and project goals. Our expert team is ready to support the success of your research.
References
- Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Molecular Cancer. 2022, 21(1): 56.
- Kangas P, Nyman T A, Metsähonkala L, et al. Towards optimised extracellular vesicle proteomics from cerebrospinal fluid. Scientific Reports. 2023, 13(1): 9564.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.