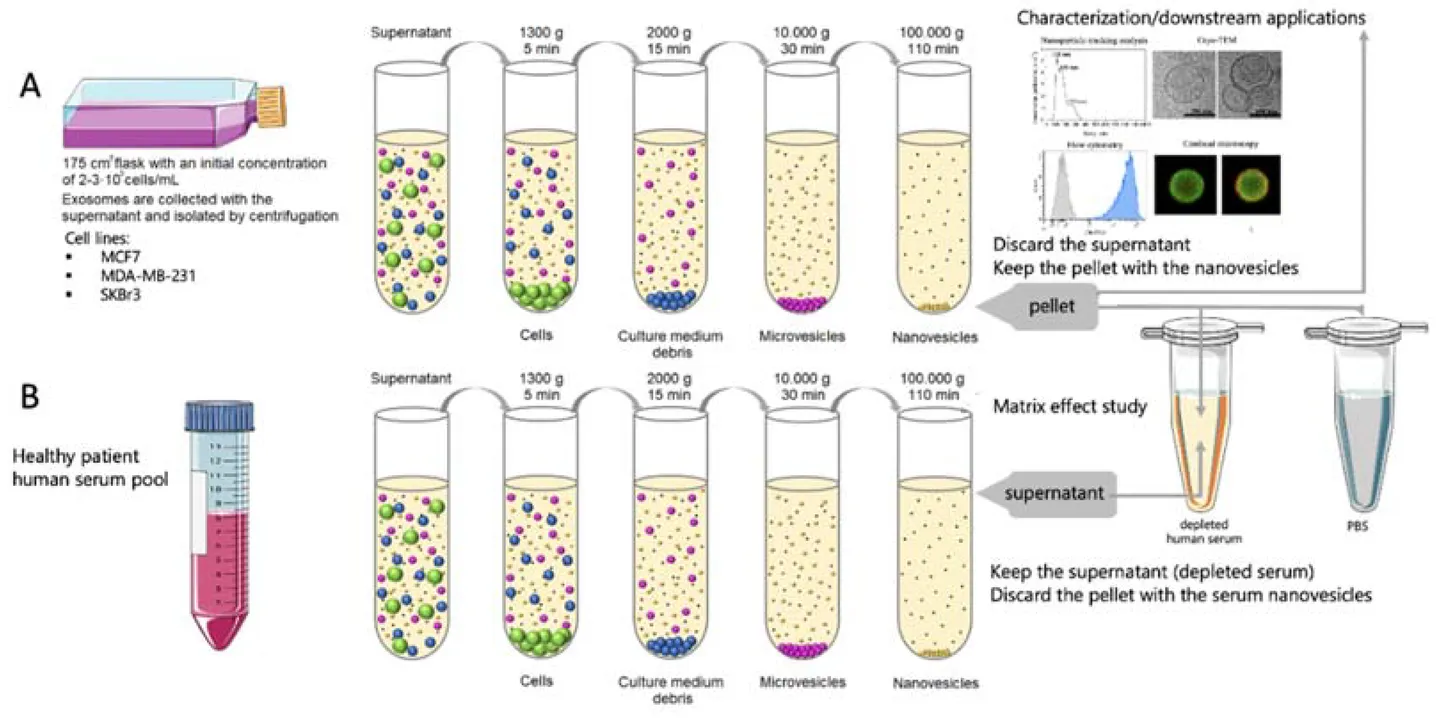
Fungal Extracellular Vesicle Isolation Service
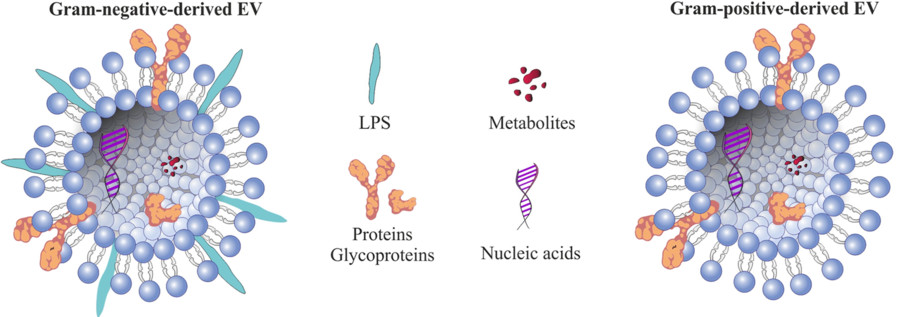
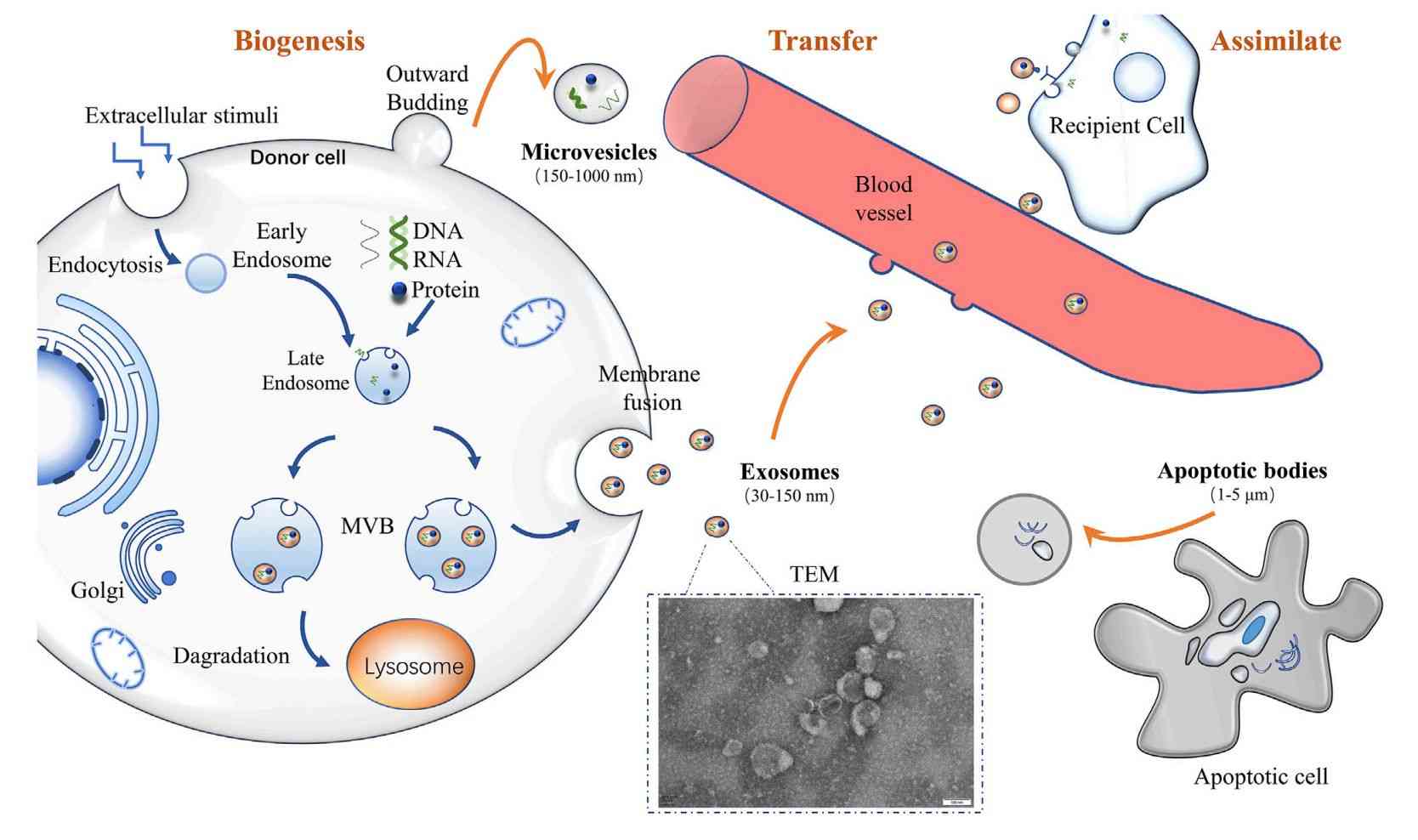
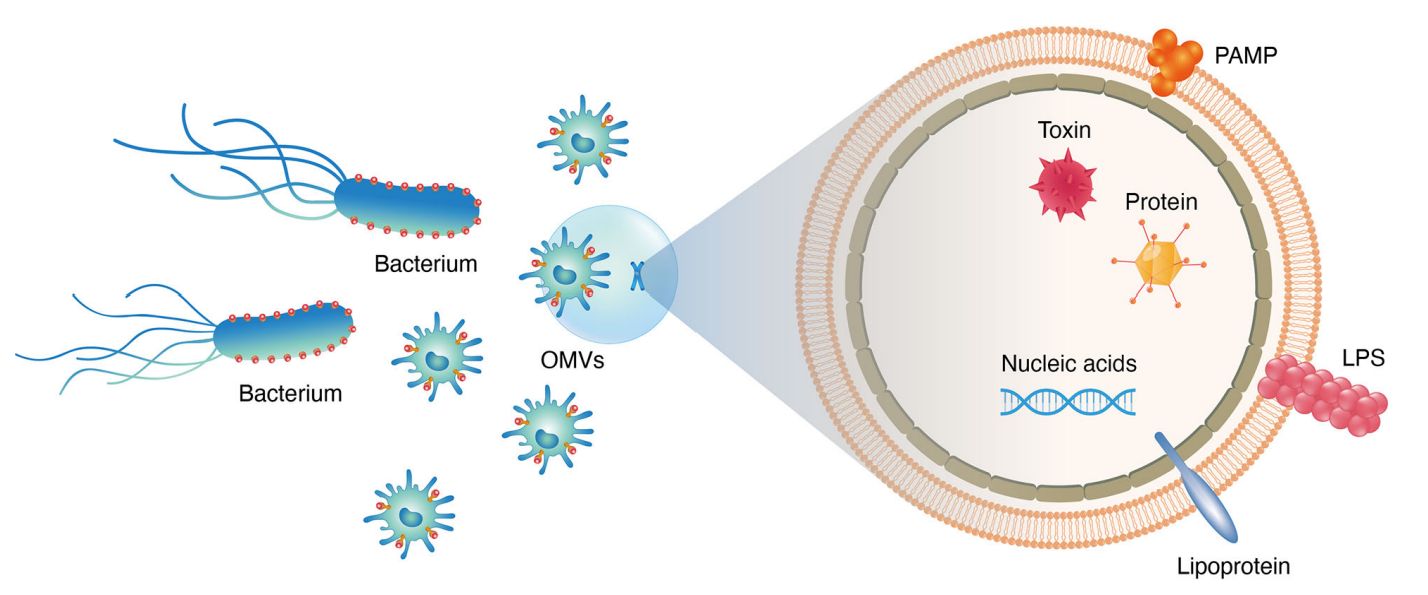
Fungal extracellular vesicles (EVs) are lipid bilayer-enclosed nanostructures released by a wide range of fungal organisms, including both environmental and pathogenic species. These vesicles serve as unconventional pathways for the export of biologically active molecules such as proteins, lipids, nucleic acids, and polysaccharides. Unlike classical secretion systems, fungal EVs can shuttle their cargo across the cell wall, a structural barrier once thought to preclude such transport. This remarkable capability underscores their functional importance in fungal physiology and host interactions.
At Creative Biostructure, we specialize in the isolation and enrichment of fungus-derived extracellular vesicles, offering custom solutions tailored to various research and application goals. Our services enable clients to explore the molecular composition, immunological activity, and translational potential of fungal EVs with high accuracy and consistency.
Why Study Fungal Extracellular Vesicles?
Fungal EVs are gaining increasing attention due to their multifaceted biological roles and translational potential in both human health and agricultural systems:
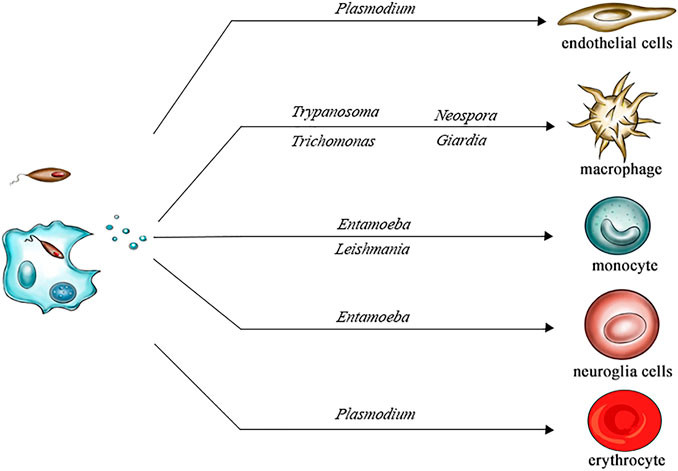
- Host immune interaction: Fungal EVs can modulate innate immune responses by activating or suppressing macrophages and dendritic cells depending on their molecular composition.
- Virulence factor delivery: Many known fungal virulence-associated proteins, including enzymes and capsular components, are exported via EVs and contribute to fungal pathogenicity.
- Stress adaptation and communication: EVs play roles in environmental sensing, intracellular communication, and fungal survival under hostile conditions.
- Biomarker discovery and vaccine research: The antigenic properties of fungal EVs make them promising candidates for non-invasive diagnostics and immunotherapeutic strategies.
Given their diverse and complex cargo, including metabolic enzymes, lipids, polysaccharides, and small RNAs, fungal EVs provide a valuable perspective on fungal biology and pathogenesis. Studying these vesicles may lead to the identification of new antifungal targets, diagnostic biomarkers, and immunomodulatory therapeutic strategies.
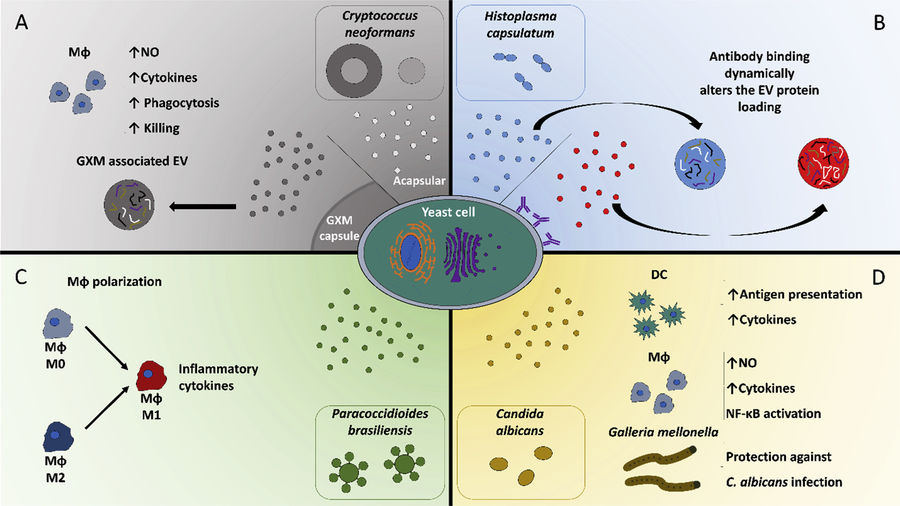 Figure 1. Interactions Between Fungal Extracellular Vesicles and Host Immune Responses. (Zamith-Miranda D, et al., 2018)
Figure 1. Interactions Between Fungal Extracellular Vesicles and Host Immune Responses. (Zamith-Miranda D, et al., 2018)
Our Fungus-Derived Extracellular Vesicle Isolation Techniques
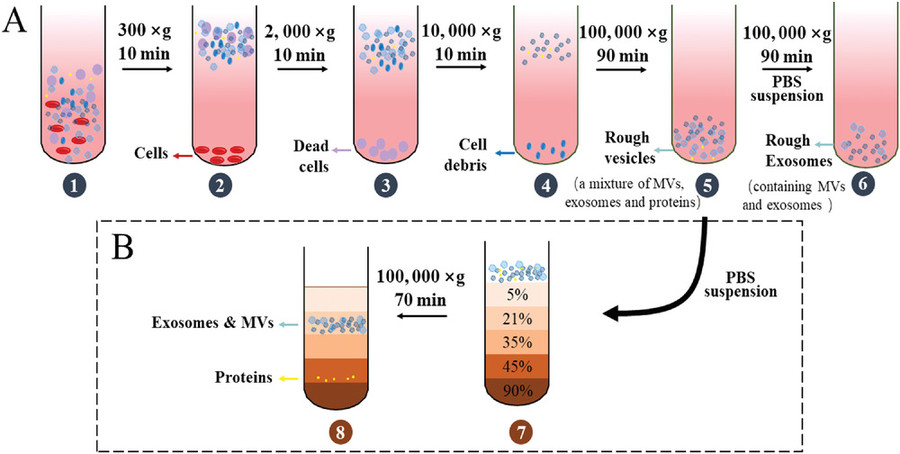
To accommodate diverse research needs and fungal species, Creative Biostructure offers multiple isolation strategies. Each method is optimized to balance yield, purity, and compatibility with downstream applications.
| Technique | Description | Recommended Applications |
|---|---|---|
| Differential Ultracentrifugation | Sequential centrifugation steps that isolate vesicles based on size and density. | General-purpose isolation; suitable for most fungal culture supernatants. |
| Density Gradient Centrifugation | Separation of vesicles using sucrose or iodixanol gradients to improve purity. | Ideal for proteomic or RNA-seq applications requiring high purity. |
| Ultrafiltration (UF) | Size-based filtration using membranes with defined molecular weight cutoffs. | Rapid processing of large volumes; useful for culture media with low vesicle concentration. |
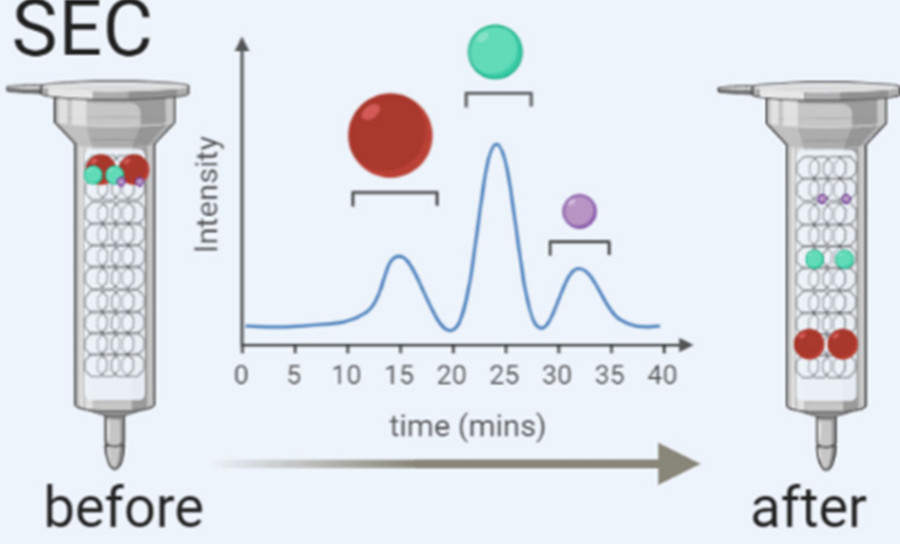
| Size Exclusion Chromatography (SEC) | Gentle purification based on vesicle size using porous resin columns. | Suitable for functional studies; preserves vesicle integrity. |
| Immunoaffinity Capture | Use of antibodies targeting fungal exosome surface markers for selective isolation. | Targeted isolation of specific vesicle subpopulations or biomarker discovery. |
| Polymer-Based Precipitation | Use of polymers like PEG to aggregate and precipitate vesicles from solution. | High-throughput or preliminary studies where speed is prioritized. |
We provide customized isolation workflows that strategically combine various techniques to enhance EV yield, purity, and biological activity according to your specific research objectives.
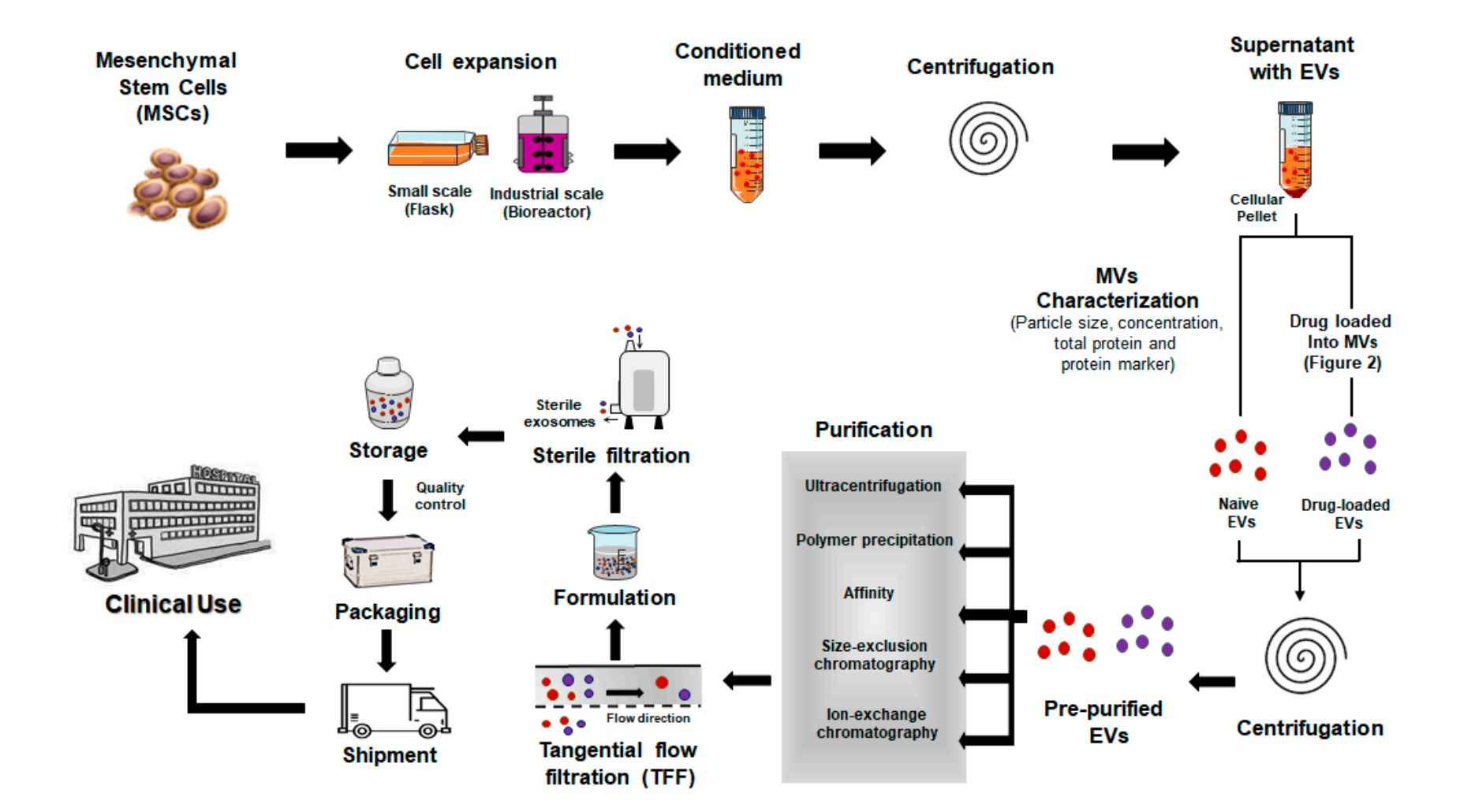
Our Fungal Extracellular Vesicle Isolation Workflow
At Creative Biostructure, we offer a robust and customizable workflow for isolating EVs from diverse fungal species and morphologies. Our approach is optimized to accommodate the unique features of fungal EVs, including their variable size, cargo complexity, and biogenesis pathways, ensuring reproducibility and compatibility with downstream omics and immunological assays.
The standard workflow includes the following steps:
Fungal Cultivation
Fungal strains are cultured in defined media with tailored pH and carbon sources to trigger vesicle release, inducing hyphal or yeast forms as needed.
Supernatant Harvesting
Supernatants are harvested at mid-late growth, then cleared by stepwise centrifugation and filtration to ensure pure, high-quality starting material.
EV Concentration and Isolation
EVs are enriched by ultrafiltration and ultracentrifugation, with optional SEC, density gradients, or immunoaffinity capture for purity and subtype specificity.
Optional Quality Assessment
Characterization includes DLS/NTA for size and concentration, TEM for morphology, LC-MS/MS for proteomics, and Western blotting of fungal EV proteins.
Result Delivery
Clients receive validated fungal EVs with a full report on yield, size distribution, and QC metrics, ready for downstream functional, immunological, or molecular studies.
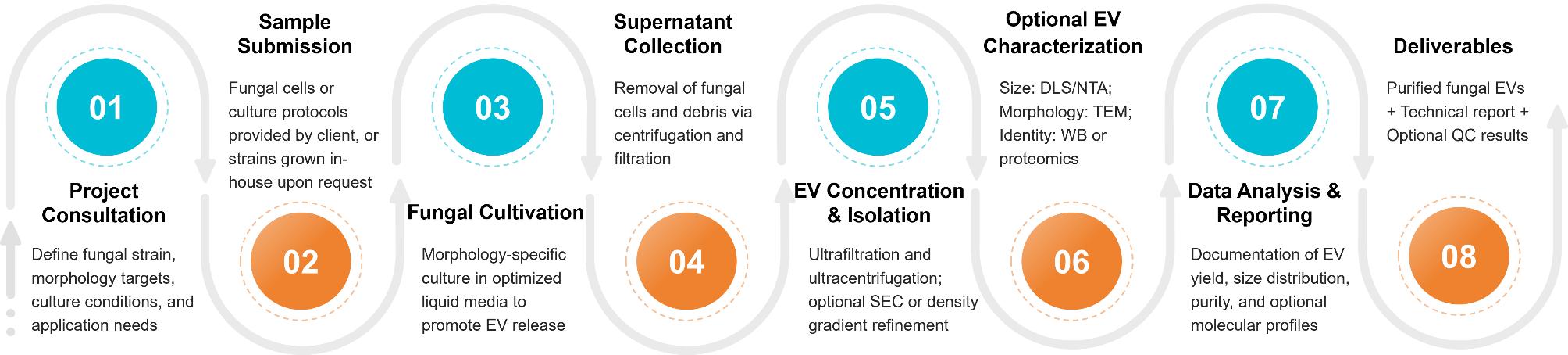 Figure 2. Fungal Extracellular Vesicle Isolation Project Workflow. (Creative Biostructure)
Figure 2. Fungal Extracellular Vesicle Isolation Project Workflow. (Creative Biostructure)
Sample Requirements
To support reliable and reproducible fungal EV isolation, we accept the following sample types:
- Sterile liquid culture supernatants collected from fungal strains under optimized conditions (preferred format)
- Pre-filtered media stored at 4°C for short-term or at -80°C for long-term preservation
- Minimum volume: 20-50 mL, depending on expected vesicle yield and analysis scope
Prior to sending your samples, please consult with our technical team for guidance on collection, storage, and shipping to ensure vesicle integrity.
Quality Control and Deliverables
Each project includes standard quality checks to confirm the presence and physical characteristics of fungal extracellular vesicles. Advanced analytical options are available upon request to support in-depth molecular profiling.
Your deliverables will include:
- Purified fungal EVs, ready for downstream applications
- Summary report detailing vesicle concentration, size range, and isolation method
- Optional data outputs, such as TEM visualization, protein content, or RNA quantification
- Full technical documentation outlining methods, findings, and storage recommendations
Case Study
Case: Isolation and Characterization of Candida albicans EVs
Researchers isolated and analyzed extracellular vesicles secreted by C. albicans in both yeast and hyphal morphologies to uncover functional differences linked to pathogenicity.
Isolation Method
EVs were obtained through differential centrifugation, 0.45 µm filtration, ultrafiltration (100 kDa), and ultracentrifugation at 100,000 × g. The workflow ensured high-purity vesicle samples for downstream analysis.
Key Insights
- Size Profile: Hyphal EVs (HEVs) were smaller (100-200 nm) and more heterogeneous than yeast EVs (YEVs), which ranged mostly from 400-500 nm.
- Cargo Complexity: HEVs carried 1,598 proteins versus 264 in YEVs, including components of the ESCRT pathway and an active 20S proteasome.
- Biological Relevance: Only YEVs restored growth in a cell wall mutant under stress, while HEVs triggered TNF-α release and macrophage cytotoxicity.
Conclusion
This study highlights morphology-dependent EV differences in C. albicans, emphasizing the importance of precise isolation methods for capturing vesicle diversity and functional relevance in host-pathogen studies.
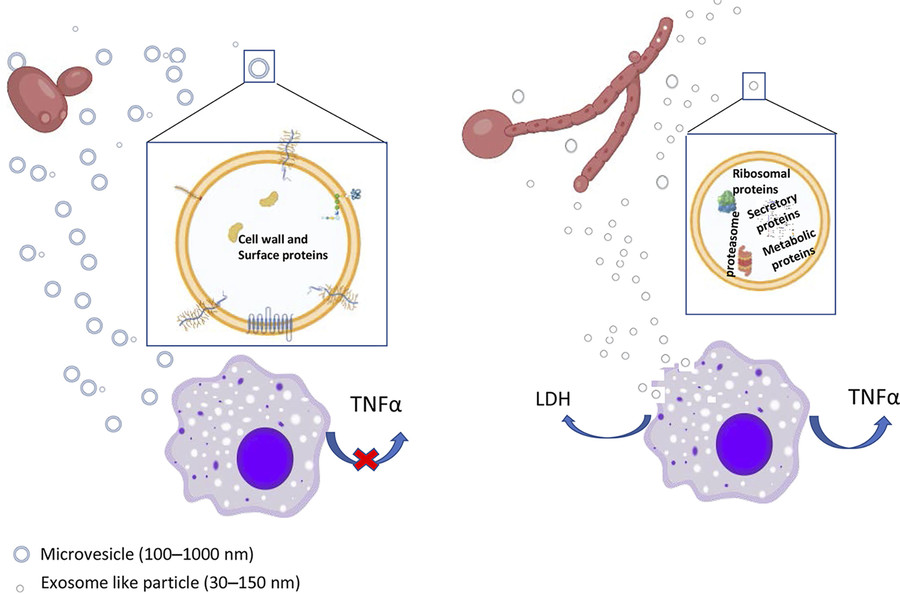 Figure 3. Comparison of HEVs and YEVs: Protein Cargo and Macrophage Interaction. (Martínez-López R, et al., 2022)
Figure 3. Comparison of HEVs and YEVs: Protein Cargo and Macrophage Interaction. (Martínez-López R, et al., 2022)
Ready to accelerate your fungal EV research? Creative Biostructure delivers high-quality, customizable isolation solutions tailored to your scientific goals. Contact us to discuss your project requirements and request a quote.
References
- Zamith-Miranda D, Nimrichter L, Rodrigues M L, et al. Fungal extracellular vesicles: modulating host–pathogen interactions by both the fungus and the host. Microbes and Infection. 2018, 20(9-10): 501-504.
- de Toledo Martins S, Szwarc P, Goldenberg S, et al. Extracellular vesicles in fungi: composition and functions. Fungal Physiology and Immunopathogenesis. 2018: 45-59.
- Martínez-López R, Hernáez M L, Redondo E, et al. Candida albicans hyphal extracellular vesicles are different from yeast ones, carrying an active proteasome complex and showing a different role in host immune response. Microbiology Spectrum. 2022, 10(3): e00698-22.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.