Infectious Disease Exosome Solutions
In infectious disease biology, exosomes play a critical role in pathogenesis and immunity. Pathogens utilize the exosome biogenesis pathway to facilitate viral exit and immune evasion, while the host utilizes these vesicles to coordinate antimicrobial responses.
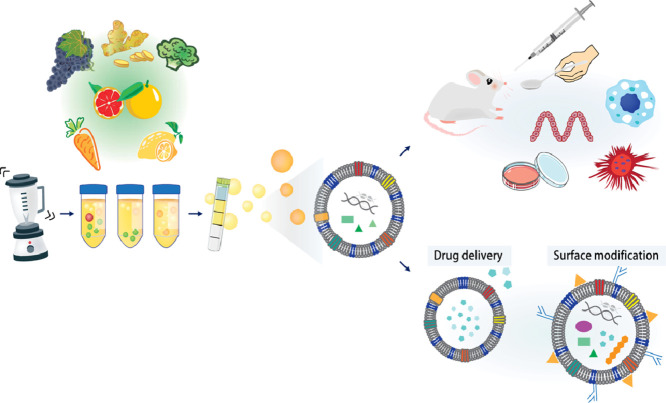
We provide end-to-end infectious disease exosome solutions. Whether you are characterizing exosomes and microvesicles involved in viral transmission (e.g., HIV, SARS-CoV-2), isolating Bacterial Membrane Vesicles (OMVs) for virulence studies, or developing exosome-based vaccines, our platform provides the precise isolation and profiling tools needed to investigate these complex biological processes.
Exosomes in Infection Biology
Why is exosomes infectious disease research critical? These vesicles function as key mediators of intercellular communication during infection.
- Viral Transmission & Evasion: Many viruses exploit the host's exosomal machinery to exit cells. These "hijacked" exosomes can carry viral RNA or proteins to uninfected cells, facilitating infection spread while shielding the pathogen from neutralizing antibodies.
- Bacterial Virulence Factors: Bacteria release Outer Membrane Vesicles (OMVs) carrying toxins, DNA, and virulence factors to manipulate host cells and modulate the immune response. Researching OMVs is essential for understanding sepsis and bacterial pathogenesis.
- Immune Modulation: Infected host cells release exosomes that can either trigger pro-inflammatory responses (cytokine release) or induce immune suppression, influencing the clinical outcome of the infection.
- Therapeutic Potential: Engineered exosomes are being developed as treatments for infectious diseases, acting as delivery vehicles for antiviral agents or as novel, cell-free vaccine platforms.
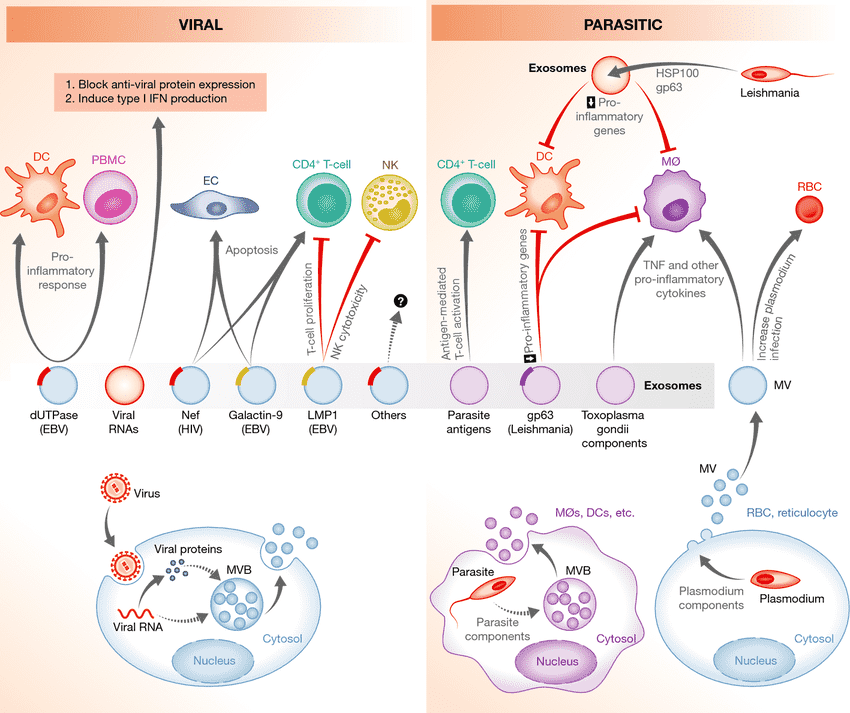 Figure 1. Exosomes from virus- or parasite-infected cells can stimulate or inhibit T-cell activation, modulating both innate and adaptive immunity. (Schorey JS, et al., 2015)
Figure 1. Exosomes from virus- or parasite-infected cells can stimulate or inhibit T-cell activation, modulating both innate and adaptive immunity. (Schorey JS, et al., 2015)
Our Integrated Infection Research Workflow
Researching pathogens requires specialized purification techniques to distinguish host vesicles from infectious particles. We provide rigorous workflows to ensure sample purity and safety.
| Service Pillar | Key Services & Technologies We Provide |
|---|---|
| Pathogen-Specific Isolation | High-Resolution Separation: Distinguishing exosomes from virions (which share similar sizes) is a major technical challenge. We utilize Size Exclusion Chromatography (SEC) and Density Gradient Ultracentrifugation to effectively fractionate non-infectious exosomes from viral particles. We also specialize in isolating Bacterial OMVs. |
| Pathogen Cargo Profiling | Pathogen-Derived Cargo Analysis: We profile the vesicular content to identify pathogen-specific components. This includes Viral RNA Sequencing to detect viral transcripts and Proteomics to identify bacterial toxins or viral envelope proteins on the vesicle surface. |
| Immune Functional Assays | Host Response Analysis: We validate the immunomodulatory effects of exosomes. Utilizing Flow Cytometry and Cytokine Arrays, we measure T-cell activation, macrophage polarization, and inflammatory cytokine release. |
| Vaccine Engineering | Antigen Display: We support exosome vaccine development by engineering exosomes to display viral antigens (e.g., Spike protein, Env) on their surface, creating stable, highly immunogenic candidates. |
Infectious Disease Applications We Support
Our platform covers the three main areas of infection research: Viral Pathogenesis, Bacterial Virulence, and Therapeutic Intervention.
Viral and Bacterial Infection Exosome Research
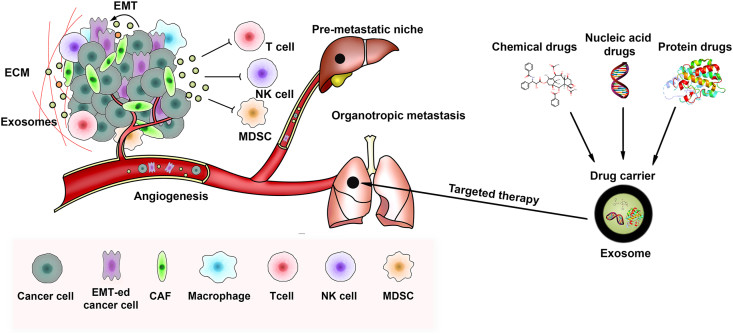
We help you investigate host-pathogen interactions at the molecular level. For viral studies, we distinguish between infectious virions and host-derived exosomes to understand how viruses alter the host secretome. For bacteriology, we characterize Outer Membrane Vesicles (OMVs) from Gram-negative bacteria, analyzing their role in biofilm formation and antibiotic resistance transfer.
Immune Response Modulation via Exosomes
In conditions like Sepsis or Acute Respiratory Distress Syndrome (ARDS), exosomes drive systemic inflammation. We isolate circulating exosomes from patient plasma or infection models and test their ability to induce pro-inflammatory cytokines (IL-6, TNF-α) in recipient monocytes, helping you map the inflammatory cascade.
Exosome-Based Vaccine & Therapeutic Development
We leverage exosomes as a vaccine platform. We assist in loading viral antigens or mRNA into exosomes to create vaccine candidates that mimic natural infection. We also test exosomes treatment of infectious diseases, such as using MSC exosomes to modulate the immune response in sepsis models.
Advantages of Our Infectious Disease Platform
Infection research poses specific technical hurdles regarding purification purity and sample safety. We have packaged our technical expertise into specific service modules designed to solve these pathogen-specific challenges.
Virion-Exosome Separation Services
Viruses (e.g., HIV, Influenza) and exosomes are both lipid vesicles of similar size (~100nm), causing standard precipitation kits to co-isolate them and compromise data integrity. We solve this with our specialized Density Gradient Ultracentrifugation and SEC workflows. By exploiting subtle differences in buoyant density and size, we achieve superior separation of host exosomes from viral particles, ensuring your transcriptomic data accurately reflects the host response rather than viral contamination.
Bacterial Membrane Vesicle (OMV) Isolation
Bacterial Outer Membrane Vesicles (OMVs) require different isolation strategies than eukaryotic exosomes to ensure they are free from bacterial debris. We offer specialized Bacterial Extracellular Vesicle Isolation Services optimized for bacterial culture supernatants. Our protocols ensure high yields of OMVs while effectively removing whole bacteria and reducing free endotoxin (LPS) contamination, facilitating clean virulence and immunogenicity studies.
Host-Pathogen Co-Culture Functional Assays
Demonstrating that exosomes mediate an infection signal requires functional proof beyond simple isolation. We provide specific Exosome Cellular Functional Assays using complex co-culture models (e.g., Infected Epithelial Cells + Immune Cells). We isolate exosomes from the infected "donor" cells and apply them to recipient immune cells, allowing you to validate that exosomes and microvesicles are the specific carriers of the pathogenic signal.
Application Spotlight: MSC Exosomes as a Treatment for Bacterial Pneumonia
This analysis highlights the therapeutic potential of exosomes in managing severe infections, demonstrating their ability to clear bacteria and modulate immune responses.
Featured Technologies:
- MSC Exosome Isolation
- Antibacterial/Phagocytosis Assays
Literature Interpretation:
Treating severe bacterial pneumonia is a major challenge due to antibiotic resistance and lung injury. Researchers investigated whether human MSC-derived exosomes could treat E. coli pneumonia. They administered these exosomes to infected mice. The results were definitive. The exosome treatment significantly improved survival rates and reduced lung injury. Mechanistically, the exosomes enhanced the ability of macrophages to phagocytose bacteria while simultaneously reducing pro-inflammatory cytokines. This study proves that exosomes can actively boost the host's immune system to fight off bacterial infections.
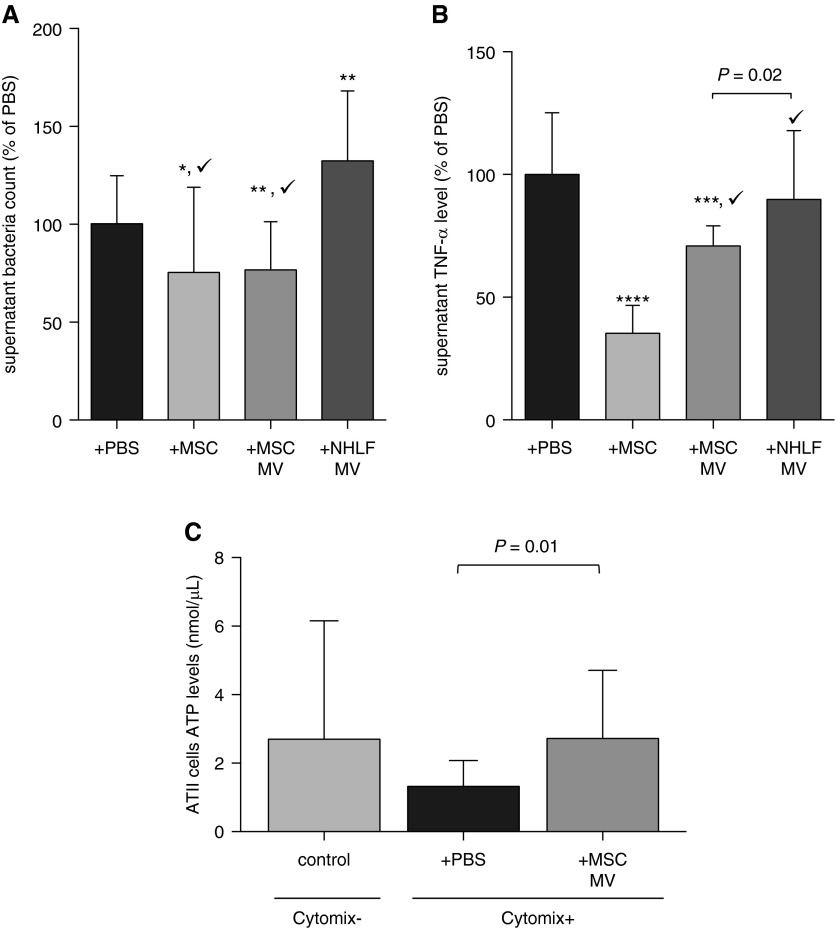 Figure 2. MSC MVs enhance bacterial clearance, reduce TNF-α secretion, and restore ATP in injured ATII cells. (Monsel A, et al., 2015)
Figure 2. MSC MVs enhance bacterial clearance, reduce TNF-α secretion, and restore ATP in injured ATII cells. (Monsel A, et al., 2015)
Start Your Infectious Disease Project
We make getting started straightforward. Our process is designed to be collaborative and transparent.
How It Works: Our Project Pathway
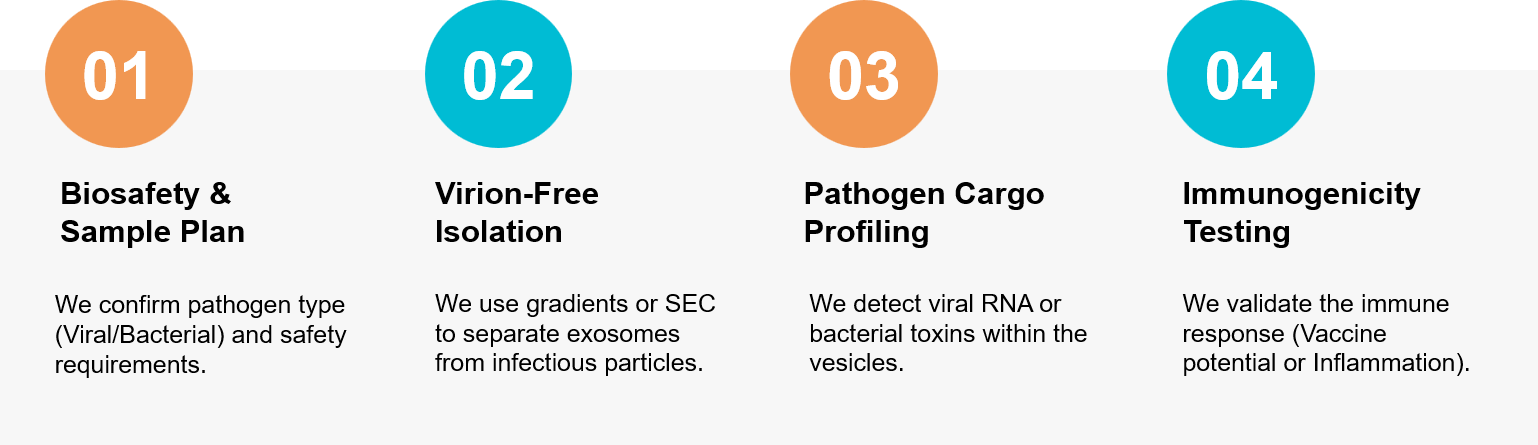 Figure 3. Our specialized workflow for separating host exosomes from pathogens and validating immune modulation. (Creative Biostructure)
Figure 3. Our specialized workflow for separating host exosomes from pathogens and validating immune modulation. (Creative Biostructure)
Ready to advance your vaccine or pathogenesis research? Our scientific team is available for a free consultation to discuss your infectious disease exosome strategy.
References
- Schorey JS, Cheng Y, Singh PP, et al. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015 Jan;16(1):24-43.
- Monsel A, Zhu YG, Gennai S, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Am J Respir Crit Care Med. 2015 Aug 1;192(3):324-36.