Exosome Drug Release and Transcytosis Assays
Engineering exosomes as refined biological nanoplatforms for drug delivery requires rigorous, quantitative validation. Before assessing bioactivity, you must first validate the performance and pharmacokinetics of your exosome vehicle. Key questions include: does the exosome release its cargo as intended? And can it successfully transport that cargo across critical biological barriers?
Our Exosome Drug Release and Transcytosis Assays are designed to answer these fundamental questions. This service platform is exclusively focused on characterizing exosomes as drug delivery vehicles, providing the critical in vitro data on release kinetics and barrier-crossing efficiency needed to advance your therapeutic program.
Why Test Release and Transcytosis First?
An effective exosome based drug delivery strategy—whether for exosome drug delivery cancer models or for neurodegenerative conditions—must prove its stability and transport capabilities.
- Validate Cargo Stability: You must prove your therapeutic cargo (e.g., small molecule, siRNA, protein) remains securely encapsulated during transit and is released in a controlled manner, not "dumped" all at once.
- Prove Transport Efficacy: For many targets (e.g., brain, oral delivery), the primary advantage of an exosome is its ability to cross physiological barriers. This transport capability must be quantified. Our assays provide the hard data to validate platforms like bovine milk-derived exosomes for drug delivery (for intestinal transport) or targeted exosomes for Parkinson's disease therapy.
This service provides the essential transport (PK) data, a critical prerequisite before testing for biological activity (Pharmacodynamics).
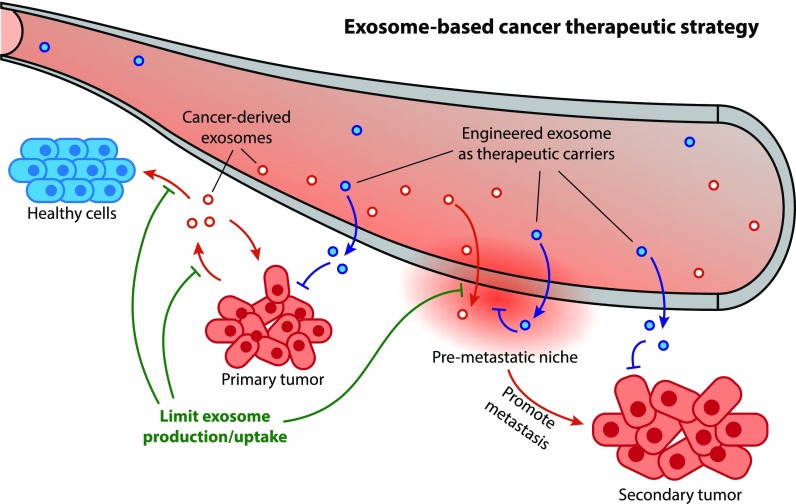
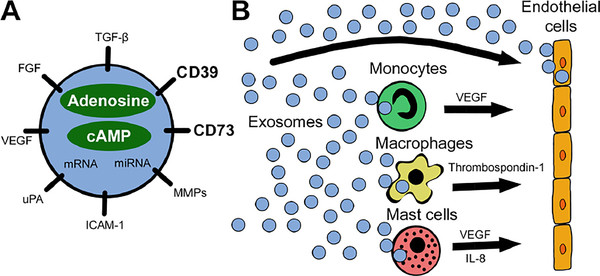
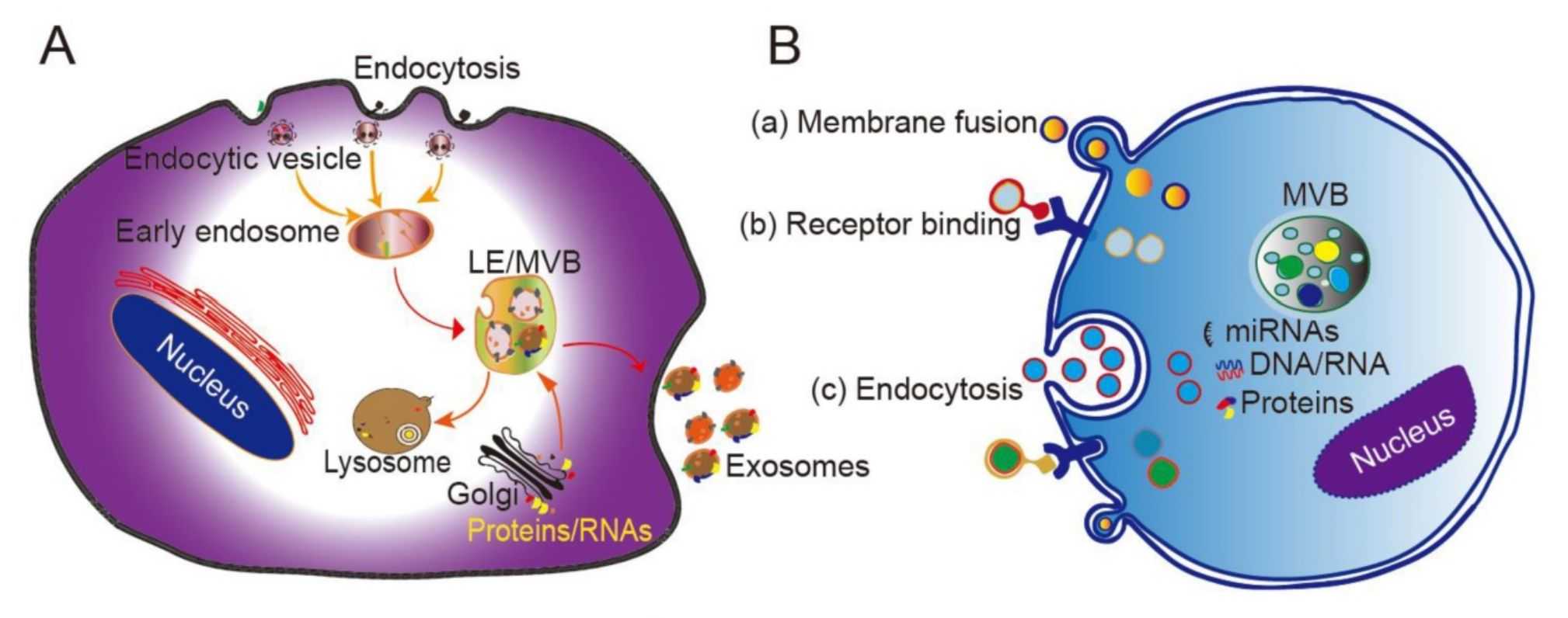
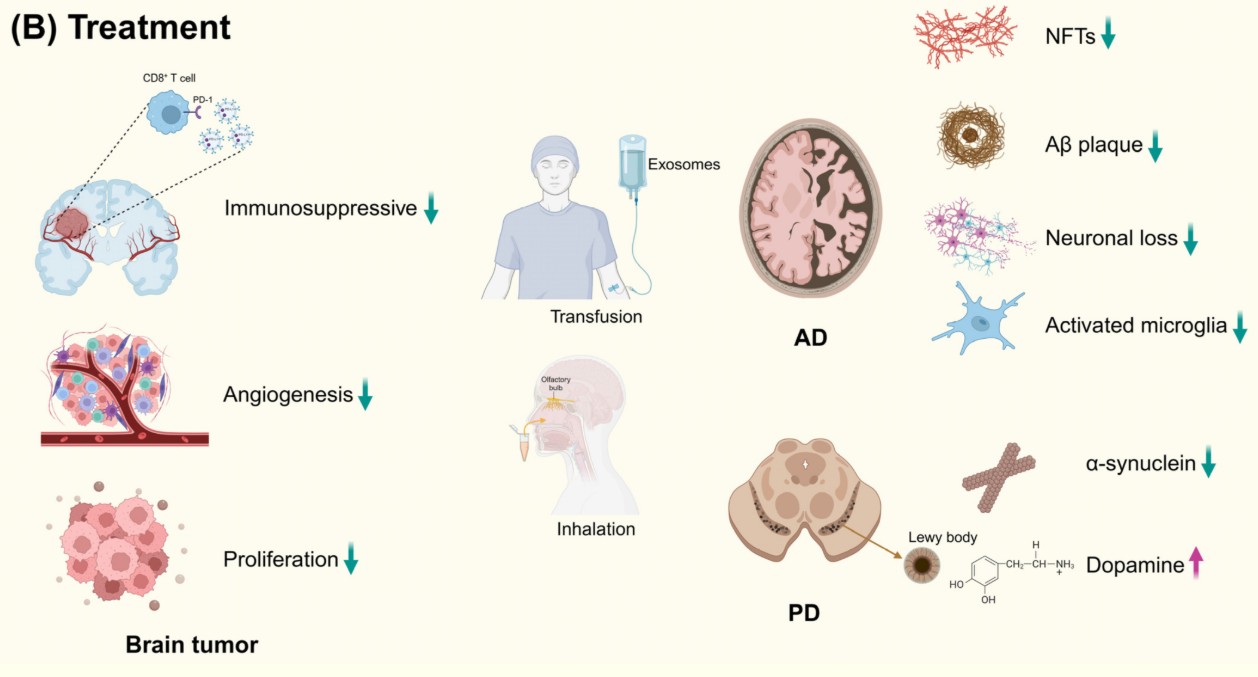 Figure 1. Exosomes, secreted by almost all cells, can cross the blood-brain barrier. Consequently, they are widely used in the treatment of brain tumors, AD, and PD. Abbreviations: AD, Alzheimer's disease; PD, Parkinson's disease; Aβ, amyloid β-protein. (Li J, et al., 2024)
Figure 1. Exosomes, secreted by almost all cells, can cross the blood-brain barrier. Consequently, they are widely used in the treatment of brain tumors, AD, and PD. Abbreviations: AD, Alzheimer's disease; PD, Parkinson's disease; Aβ, amyloid β-protein. (Li J, et al., 2024)
Our Platform of Exosome Vehicle Validation Assays
We offer two highly specialized, quantitative assays focused strictly on the performance of your exosome drug delivery system.
1. In Vitro Drug Release Kinetic Assays
This assay quantifies the rate at which your therapeutic cargo is released from the exosome into a controlled buffer, simulating physiological conditions.
- Technology Platform: We use validated dialysis-based methods (e.g., Float-A-Lyzer dialysis cassettes) that separate the free drug from the encapsulated drug.
- Methodology:
- Your cargo-loaded exosomes are placed inside a dialysis cassette with a specific molecular weight cut-off (MWCO).
- The cassette is placed in a "sink" of release buffer (e.g., PBS at 37°C) with constant stirring.
- Samples are taken from the outside buffer at multiple time points (e.g., 0, 1, 2, 4, 8, 24, 48h).
- The amount of released drug in the samples is quantified (by HPLC, ELISA, qPCR, etc.).
- Key Deliverable: A cumulative release curve (Percentage of Release vs. Time), defining your exosome's release profile (e.g., burst release vs. sustained release).
2. In Vitro Transcytosis (Barrier-Crossing) Assays
This is the gold-standard assay to prove your exosome's ability to cross biological barriers. We build functional, tight-junction cellular barriers in Transwell systems to measure transport efficiency.
- Technology Platforms and Models:
- Blood-Brain Barrier (BBB) Model: We build a high-integrity barrier using co-cultures of human brain microvascular endothelial cells (hBMECs), pericytes, and astrocytes. This is the key assay for validating exosomes as drug delivery vehicles for Parkinson's disease therapy.
- Intestinal Barrier Model: We use highly validated Caco-2 cell monolayers, which polarize and form tight junctions that mimic the human intestinal wall. This is ideal for testing oral exosome drug delivery platforms, including milk exosomes drug delivery.
- Key QC Step: Barrier integrity is confirmed via Trans-Epithelial Electrical Resistance (TEER) measurement before every experiment. Only barriers with high TEER values are used.
- Key Deliverable: The Apparent Permeability Coefficient (Papp), a quantitative value that defines how efficiently your exosome formulation crosses the barrier, compared to a negative (free drug) control.
Our Transcytosis Assay Workflow
We employ a robust and validated workflow to ensure the integrity of our barrier models and the accuracy of your transport data. Our process integrates key quality control steps, such as TEER measurements, with high-sensitivity quantification methods to deliver reliable and reproducible results.
Key Process Details & Customization
Rigorous Controls
To ensure data integrity, every experiment includes a Free Cargo Control (to measure baseline permeability), an Empty Vehicle Control (to check for barrier interference), and a low-permeability Integrity Marker Control (e.g., Lucifer Yellow).
High-Sensitivity Quantification
We employ the most appropriate method for your cargo, including qPCR for siRNA/miRNA, ELISA or LC-MS for proteins, and HPLC or LC-MS for small molecules.
Customization
We offer advanced options such as assessing Bidirectional Transport (A-B and B-A) to study efflux mechanisms and developing Custom Time Points to match your formulation's kinetics.
Our standard workflow includes the following key steps:
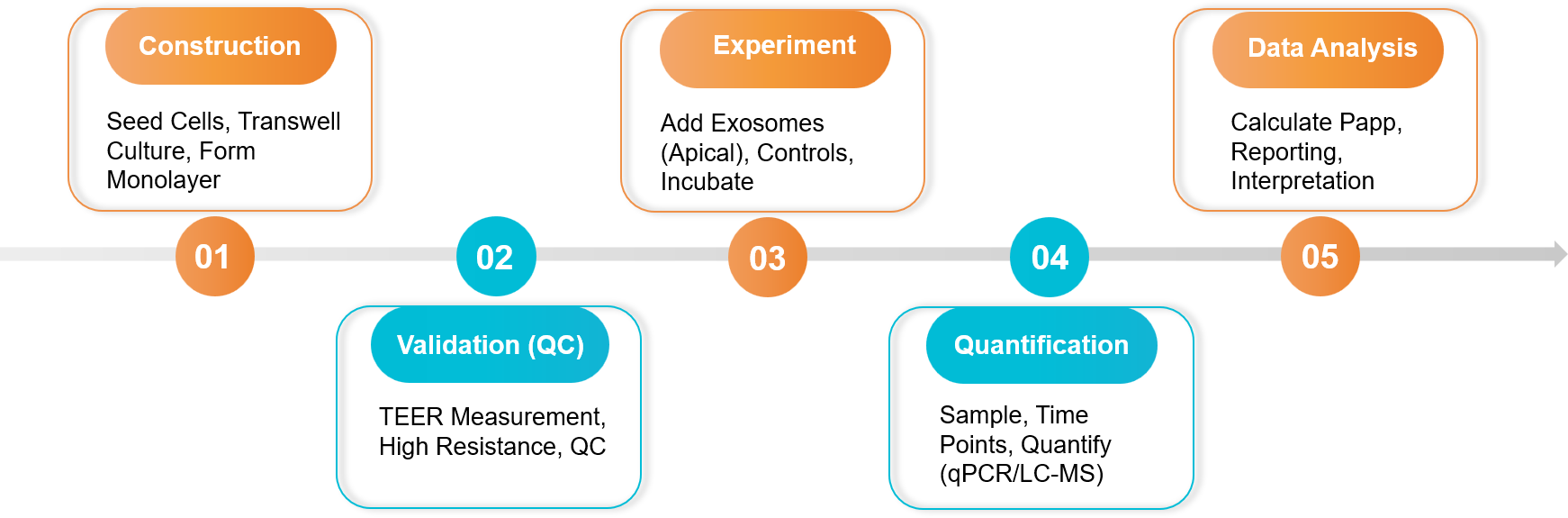 Figure 2. Exosome Drug Release and Transcytosis Assay Project Workflow. (Creative Biostructure)
Figure 2. Exosome Drug Release and Transcytosis Assay Project Workflow. (Creative Biostructure)
Sample Requirements
- Client-Provided Exosomes: Purified exosomes loaded with your specific cargo (e.g., drug, protein, siRNA).
- Client-Provided Controls (Critical):
- A sample of the "free" (non-encapsulated) cargo.
- A sample of "empty" (unloaded) exosomes.
- Cargo Information: The identity of the cargo and a validated method for its quantification (e.g., qPCR primers for siRNA, ELISA standard for protein).
Standard Deliverables
- For Release Assays: A full dataset and graph plotting cumulative cargo release (%) versus time.
- For Transcytosis Assays:
- All TEER measurement data (validating the barrier).
- A comprehensive report showing the amount of cargo transported at each time point.
- The calculated Apparent Permeability (Papp) value for your exosome formulation versus controls.
Case Study
Case: Validating Oral Delivery of Milk Exosomes Using an In Vitro Intestinal Barrier Model
Background: Oral drug delivery is limited by the intestinal barrier. Researchers tested if bovine milk-derived exosomes (mEVs) could be used as an oral exosome drug delivery system to transport a cancer prodrug (FDX) across it.
Methodology: Researchers used an In Vitro Transcytosis Assay with a Caco-2 barrier model. They added drug-loaded mEVs (FDX@mEVs) to the top (apical) side and measured the drug transported to the bottom (basolateral) side.
Key Findings: The assay confirmed mEVs provided "enhanced trans-epithelial transport". The mechanism was identified as active "FcRn-mediated transcytosis", proving the exosomes were actively "shuttled" across the barrier, not just leaking.
Conclusion: The in vitro transcytosis assay provided the critical PK validation. It proved milk exosomes drug delivery is a viable oral platform by quantifying its efficient transport across the intestinal barrier.
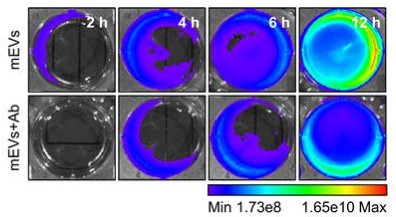 Figure 3. FcRn blockade significantly reduces the transport of mEVs to the basolateral chamber. (Jang H, et al., 2024)
Figure 3. FcRn blockade significantly reduces the transport of mEVs to the basolateral chamber. (Jang H, et al., 2024)
Ready to validate your exosome delivery vehicle? Our team will design fit-for-purpose in vitro release and transcytosis studies, confirm barrier integrity by TEER, and quantify Papp and release profiles with rigorous controls. Contact us for a free consultation and an assay plan tailored to your cargo and route.
References
- Li J, Song J, Jia L, et al. Exosomes in Central Nervous System Diseases: A Comprehensive Review of Emerging Research and Clinical Frontiers. Biomolecules. 2024 Nov 27;14(12):1519.
- Jang H, Choi J, Park D, et al. Milk-derived extracellular vesicles enable gut-to-tumor oral delivery of tumor-activated doxorubicin prodrugs. Theranostics. 2024 Aug 26;14(14):5413-5428.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.