Exosome Quantification Services
Accurate quantification of exosomes is essential for interpreting experimental results, ensuring reproducibility, and comparing findings across studies. At Creative Biostructure, we provide comprehensive exosome quantification services that follow the Minimal Information for Studies of Extracellular Vesicles (MISEV2023) guidelines. Our platform integrates particle counting, protein, lipid, and RNA quantification to deliver reliable and standardized data for both academic and industrial clients.
Why Exosome Quantification Matters
- Biological insights: Quantification establishes correlations between vesicle numbers and cellular states.
- Diagnostic applications: Enables measurement of tumor-derived or disease-specific exosomes in body fluids.
- Therapeutic development: Supports dose definition and quality control for exosome-based drug delivery systems.
- Regulatory compliance: Ensures data consistency, with results expressed in standardized units (e.g., particles/mL, µg protein, ng RNA).
Given the challenges posed by the small size, heterogeneity, and low abundance of exosomes, rigorous methodologies are necessary to achieve accuracy, reproducibility, and comparability.
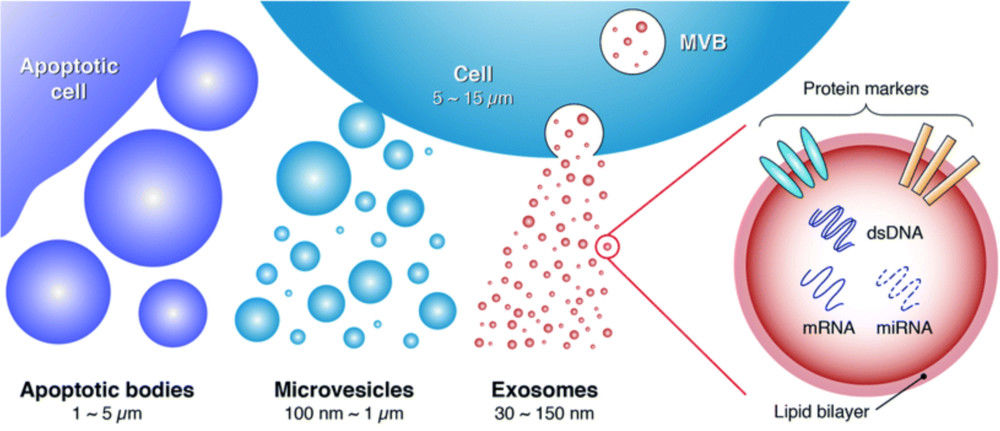
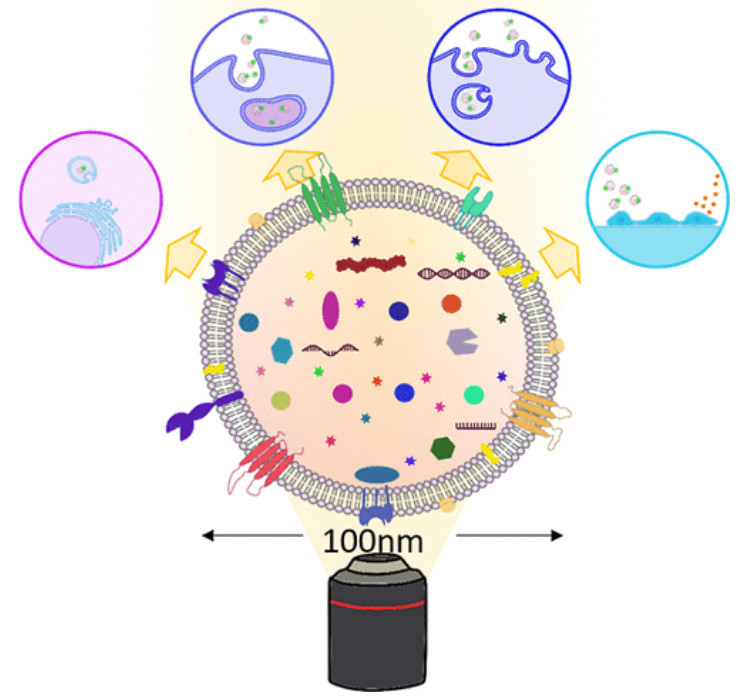
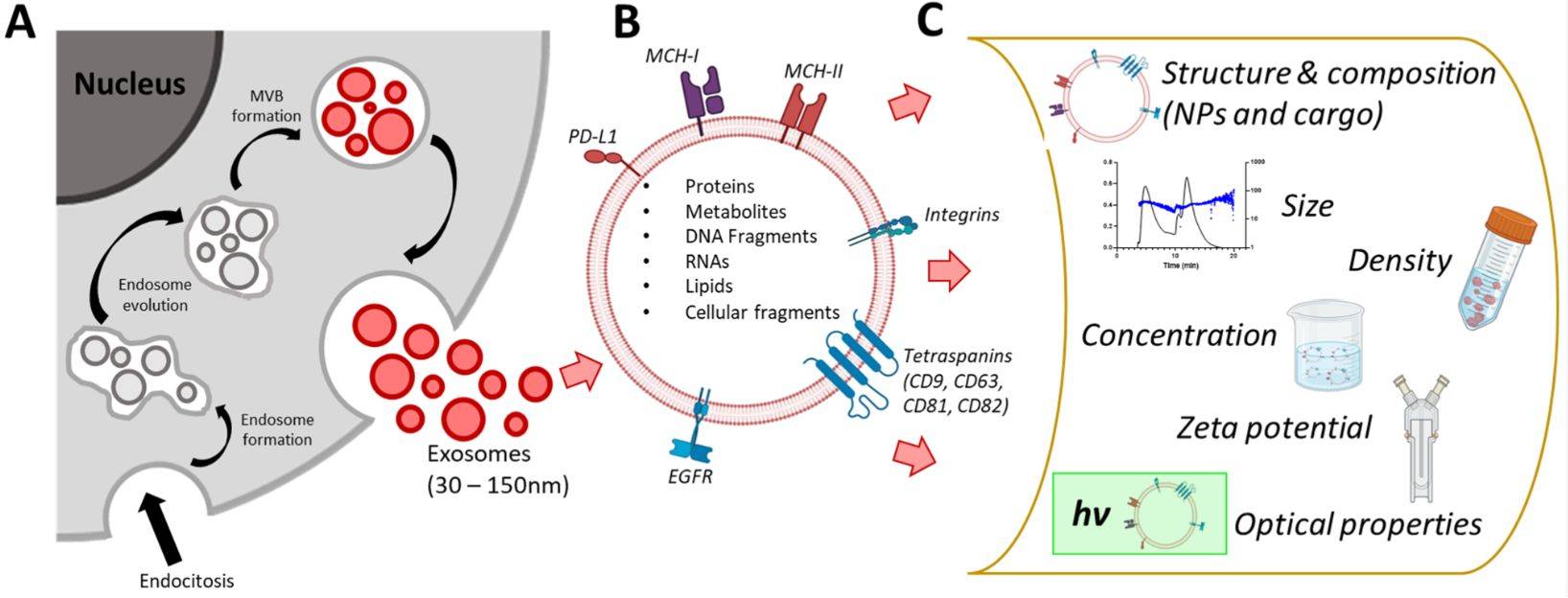
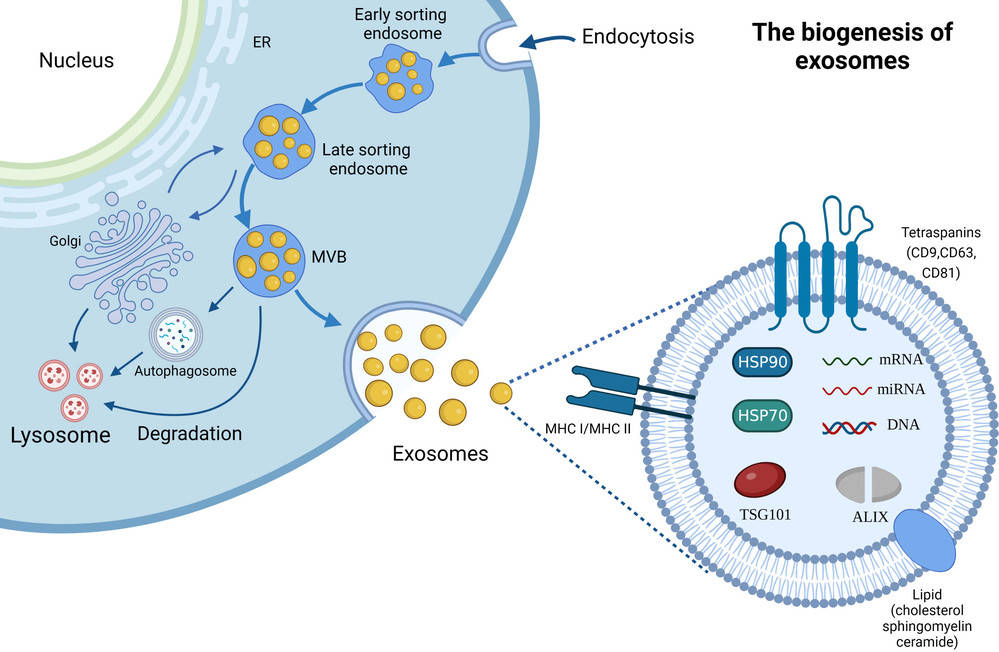 Figure 1. Biogenesis and Composition of Exosomes. (Zhang H, et al., 2023)
Figure 1. Biogenesis and Composition of Exosomes. (Zhang H, et al., 2023)
Comprehensive Quantification and Purity Assessment
A single measurement is rarely sufficient. True exosome characterization requires a multi-modal approach that quantifies not only the number of particles but also their biochemical composition. This strategy provides a complete picture of your sample and is essential for assessing its purity.
Our core philosophy involves correlating particle concentration with total protein and other molecular measurements. This allows for the calculation of critical purity metrics, such as the particle-to-protein ratio, a key parameter recommended by ISEV for distinguishing high-purity EV preparations from contaminated samples.
Our Analytically Validated Quantification Platforms
We offer a range of state-of-the-art technologies to provide a multi-dimensional view of your exosome sample. We consult with you to select the optimal methods for your specific research goals and sample type.
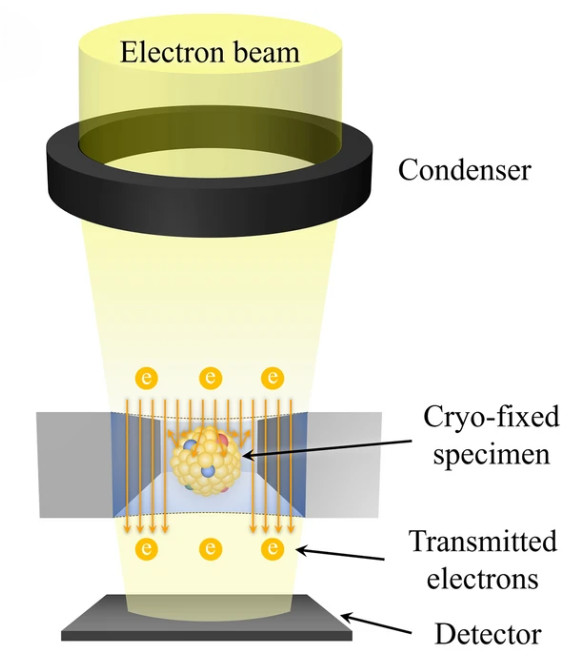
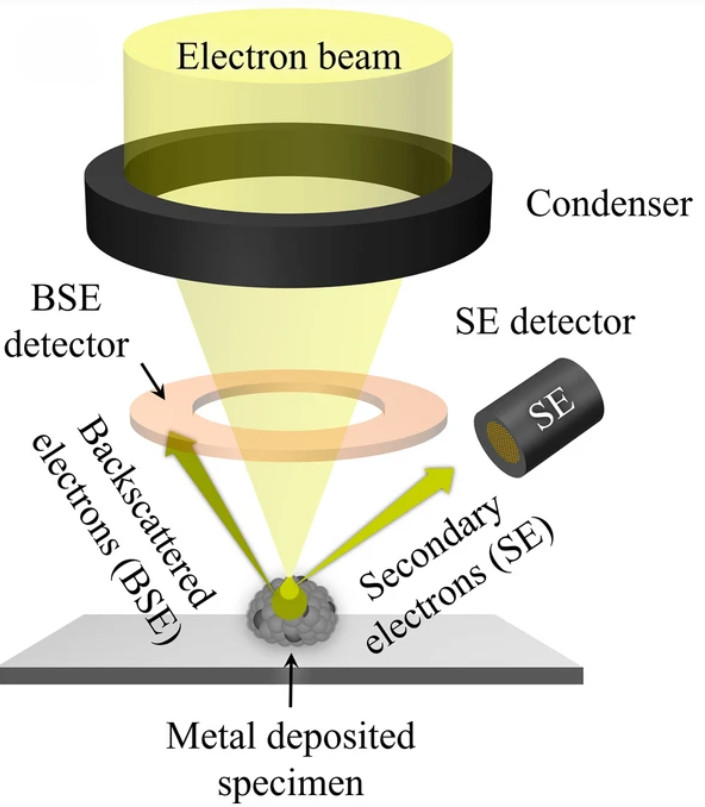
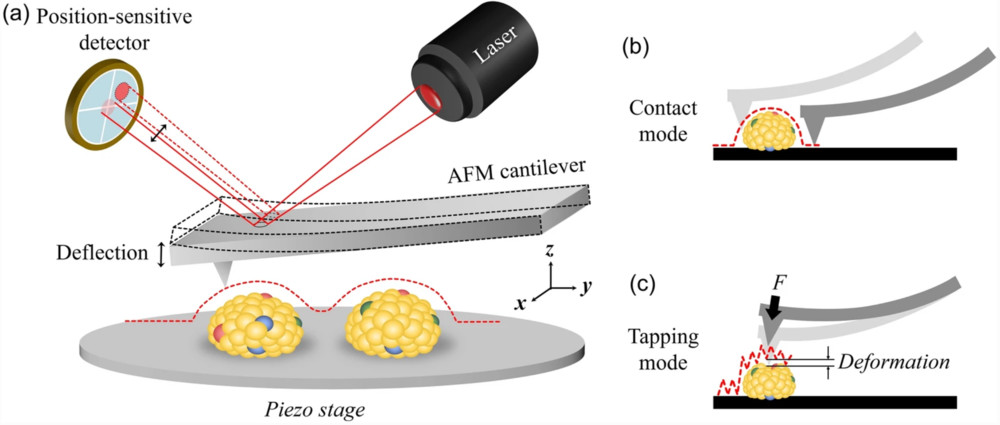
Particle Number and Size Distribution Analysis
| Technology | Principle | Key Measurements | Best For |
|---|---|---|---|
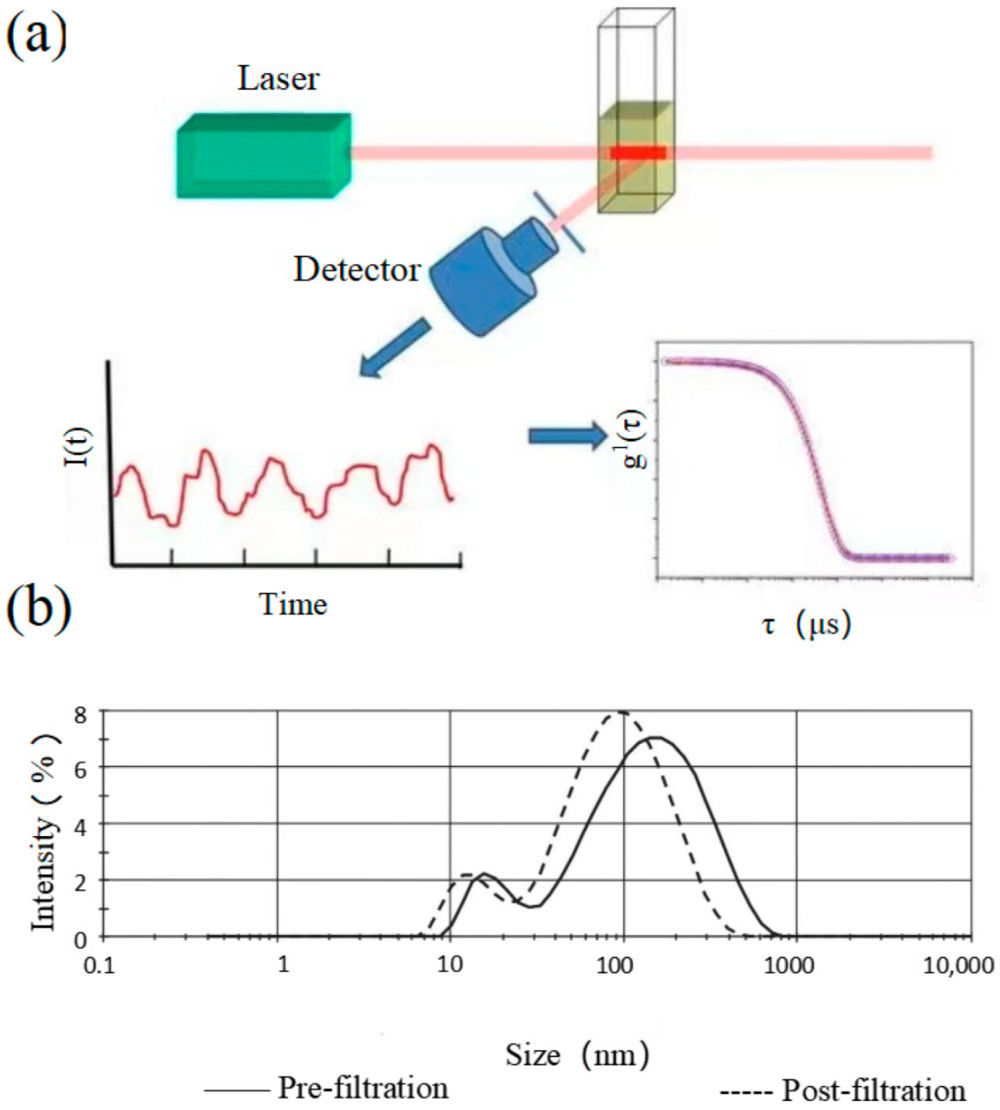
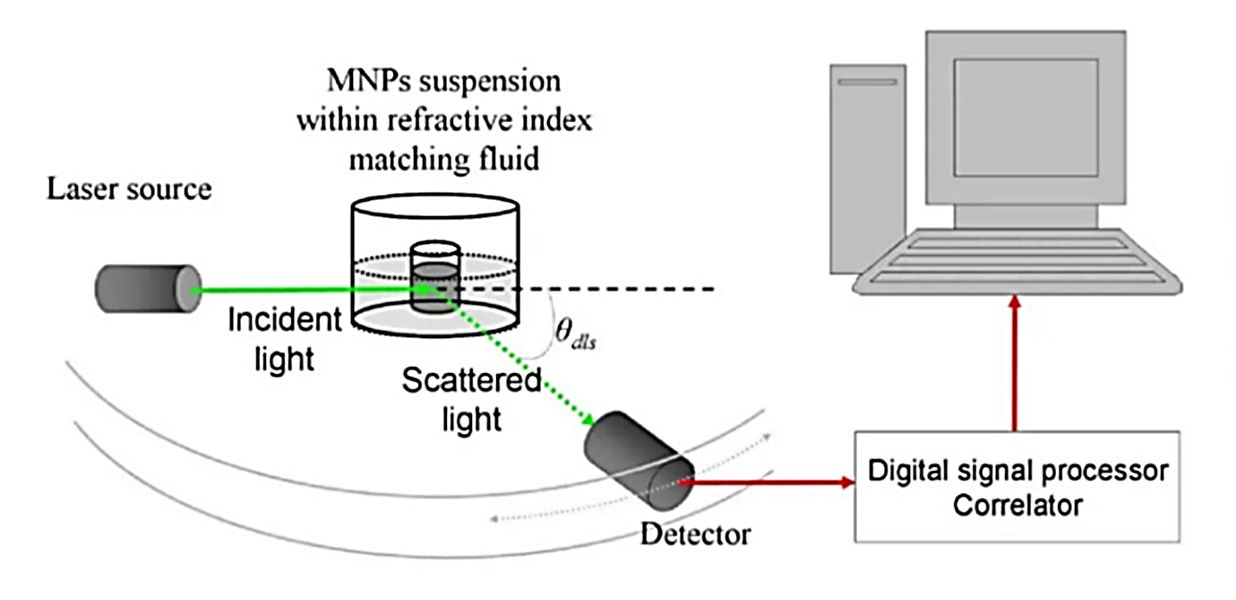
| Nanoparticle Tracking Analysis (NTA) | Visualizes and tracks the Brownian motion of individual nanoparticles to calculate their size and concentration. | Particle Concentration (particles/mL), Hydrodynamic Diameter Distribution, Mode/Mean Size | Rapid, direct measurement of overall particle concentration and size in purified samples. |
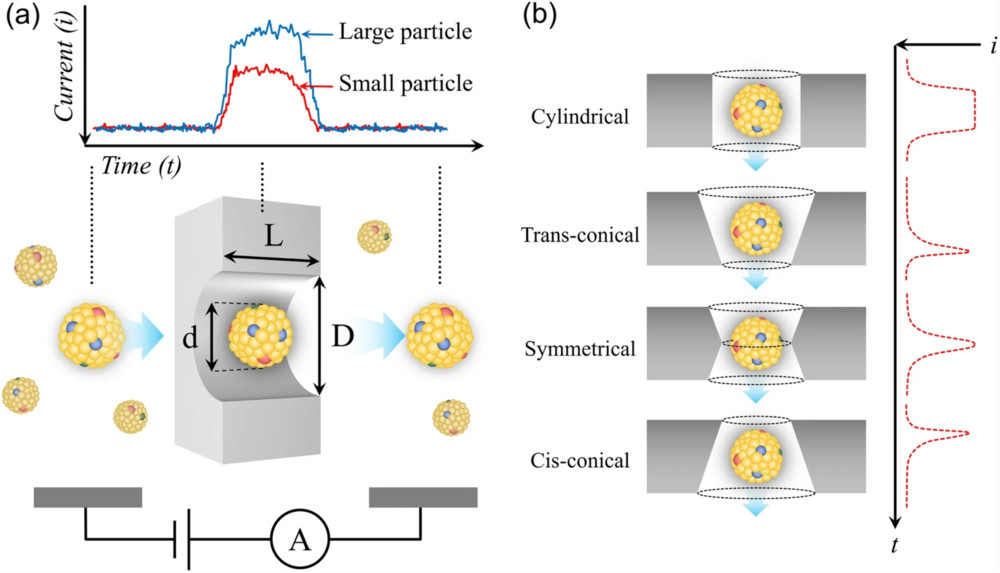
| Tunable Resistive Pulse Sensing (TRPS) | Measures the change in ionic current as single particles pass through a tunable nanopore, providing high-resolution data. | Particle Concentration (particles/mL), Particle Volume/Diameter Distribution | High-accuracy and high-resolution analysis, especially for polydisperse or complex samples. |
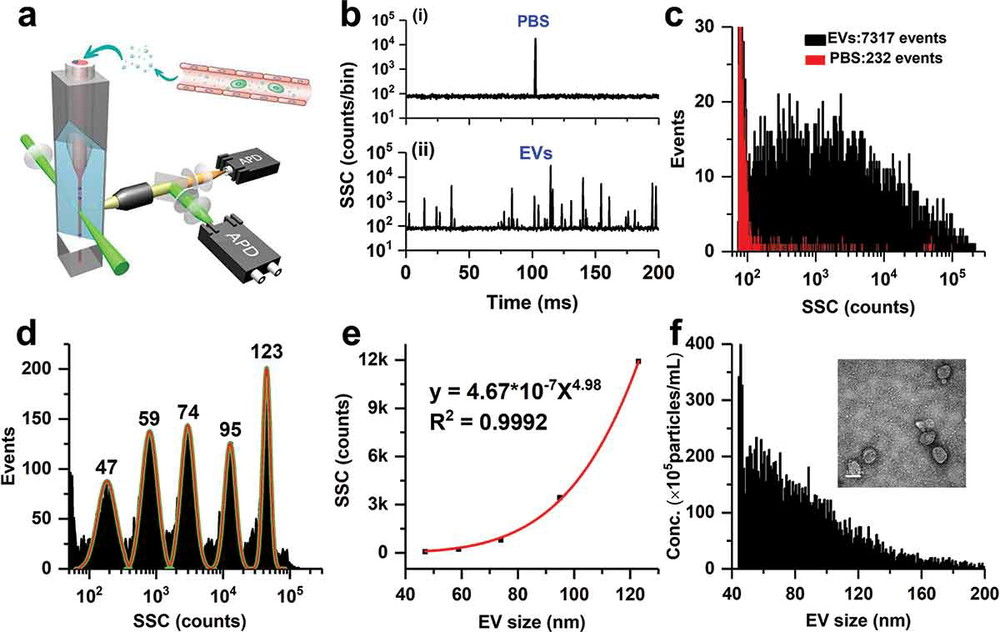
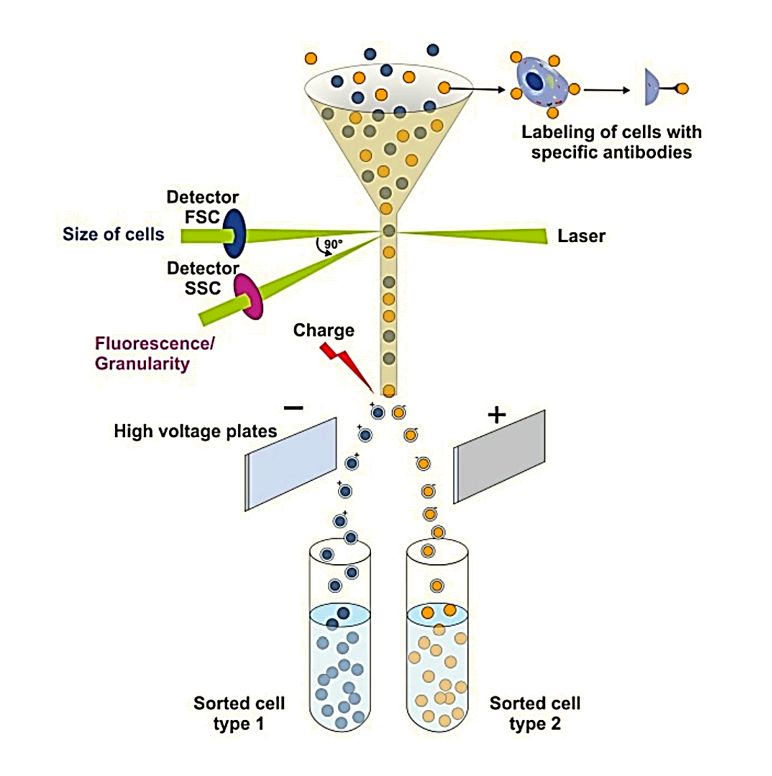
| High-Resolution Flow Cytometry (NanoFCM) | Detects light scatter from single vesicles at high throughput, enabling rapid particle counting and immunophenotyping. | Particle Count, Quantification of Specific Subpopulations (with fluorescence) | High-throughput screening and quantifying specific exosome subpopulations based on surface markers. |
Biochemical and Molecular Cargo Quantification
Total Protein Quantification
We employ sensitive colorimetric assays (e.g., BCA, Bradford) to measure the total protein content associated with your EV preparation. We clearly report whether analysis was performed on intact or lysed vesicles, as co-isolated proteins can influence results.
Total RNA and miRNA Quantification
Using high-sensitivity, dye-based fluorometric assays, such as qPCR and digital PCR (dPCR), we quantify the total RNA or specific RNA species (like miRNA) in your sample. We report on any pre-treatments (e.g., DNase) and utilize appropriate quality controls.
Total Lipid Quantification
For a complete biochemical profile, we offer specialized assays to measure the total lipid content, providing another layer of characterization.
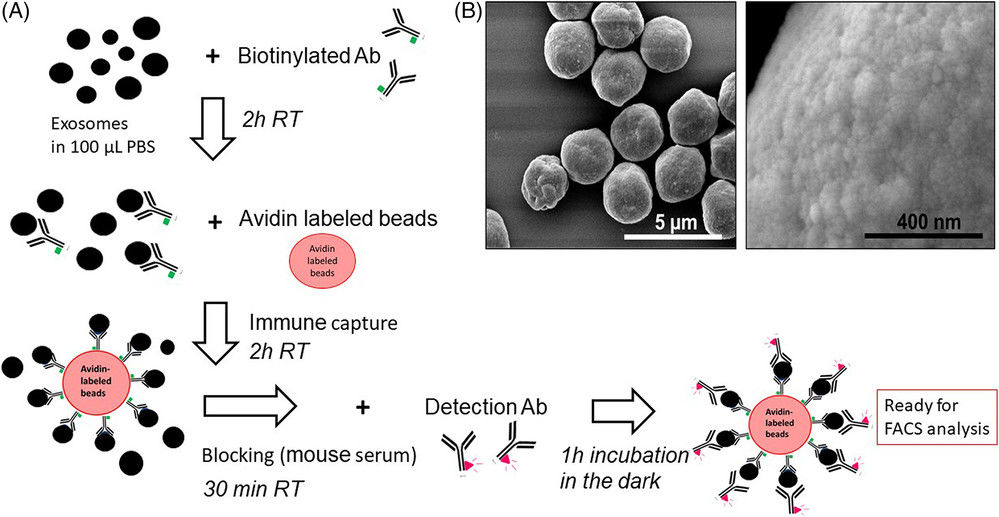
Exosome-Specific ELISA
This powerful technique quantifies exosomes based on the presence of specific surface proteins (e.g., tetraspanins CD9, CD63, CD81). It is an excellent method for measuring the concentration of specific exosome subpopulations and can be adapted for high-throughput screening.
Designing Your Quantification Strategy
The optimal quantification strategy depends on your research goals and sample type. Our team of experts provides consultations to help you design the most effective and efficient analytical plan.
- For Biomarker Discovery: A combination of a particle counting method (NTA or TRPS) with exosome-specific ELISA can provide both overall particle concentration and the concentration of a target subpopulation.
- For Therapeutic Development & QC: High-resolution methods like TRPS and NanoFCM are essential for ensuring batch-to-batch consistency in vesicle size and concentration. Total protein and RNA measurements serve as critical quality control parameters.
- For Complex Biofluids (Plasma, Serum): Orthogonal validation is crucial. We may recommend comparing data from multiple platforms to confidently parse true vesicle counts from background particles.
Standard Workflow & Timelines
| Step | Description | Estimated Time |
|---|---|---|
| Sample Receipt & QC | Check sample integrity, volume, documentation; perform pre-analysis QC | 1-2 days |
| Exosome Isolation (optional) | Vesicle purification via ultracentrifugation, SEC, or immunoaffinity | 2-4 days |
| Quantification Assays | Particle counting (NTA/TRPS/NanoFCM) and molecular profiling (protein, lipid, RNA) | 3-5 days |
| Data Analysis & Validation | Calibration curves, LoD/LoQ, reproducibility checks | 2-3 days |
| Report Delivery | Final report with raw data, statistics, standard curves, interpretation | 1-2 days |
Typical turnaround time: ~2-3 weeks (expedited service available upon request).
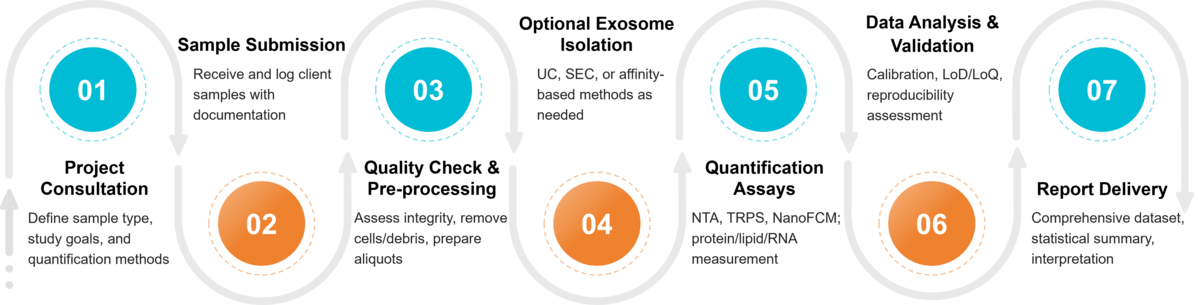 Figure 2. Project Workflow for Exosome Quantification. (Creative Biostructure)
Figure 2. Project Workflow for Exosome Quantification. (Creative Biostructure)
Sample Requirements
For reliable and reproducible exosome quantification, we recommend following the guidelines below. Please prepare samples accordingly to ensure optimal results. If isolation from raw material is needed, our Exosome Isolation Services are available to support you.
| Parameter | Requirement |
|---|---|
| Sample Types | Plasma, serum, urine, saliva, CSF, breast milk, cell culture supernatant |
| Volume | 200-500 µL (NTA/TRPS); 50-100 µL (NanoFCM); more for combined assays |
| Purity | Remove cells/debris by low-speed centrifugation before submission |
| Storage | Fresh preferred; otherwise -80 °C, aliquoted; avoid freeze-thaw cycles |
| Shipping | Ship on dry ice with clear labeling and metadata (type, method, storage) |
What Deliverables Will You Receive
Upon project completion, you will receive a comprehensive report containing:
- A detailed summary of methodologies, instrument settings, and software versions.
- Raw and processed quantitative data (e.g., particle concentration in particles/mL, protein concentration in µg/mL).
- High-resolution graphs, including particle size distribution profiles.
- Complete statistical analysis, including mean, standard deviation, and CV%.
- For quantitative assays, we provide standard curves and report on key validation metrics like LoD and LoQ.
Applications of Exosome Quantification
- Biomarker Discovery: Quantifying exosomes in biofluids to identify potential diagnostic or prognostic markers.
- Therapeutic Potency & Dosing: Standardizing exosome preparations for in vitro and in vivo studies to ensure reproducible results.
- Quality Control in Biomanufacturing: Monitoring the consistency and yield of exosome production processes.
- Exosome-Based Drug Delivery: Characterizing the concentration and purity of engineered exosomes.
- Fundamental Research: Investigating the roles of exosomes in intercellular communication by accurately measuring their secretion under various conditions.
Why Choose Creative Biostructure
- Comprehensive expertise: Over a decade of experience in exosome characterization and structural biology.
- Advanced technology platforms: Multi-method approach for cross-validation of results.
- Customized study design: Tailored workflows based on sample type, research goal, and budget.
- Strict quality control: Adherence to international standards with reproducible and validated data.
- Client-focused support: Flexible service packages for academic, biotech, and pharmaceutical partners.
Case Study
Case: Ultrasound-Stimulated Exosome Release Highlights Quantification Value
Background
Astrocyte-derived exosomes were tested as therapeutic modulators in Alzheimer's disease. Accurate quantification was central to linking vesicle yield with functional outcomes.
Methods
- Quantification: NTA showed >4-fold increase in exosome release (1.46 × 1010 → 6.06 × 1010 particles/mL).
- Validation: BCA confirmed protein rise (0.41 → 1.03 µg/µL); Western blot showed enrichment of CD63, CD9, TSG101; TEM confirmed intact morphology.
Results
- Neuronal amyloid-β uptake decreased by 26.3%.
- Exosomes rescued cell viability and lowered plaque burden in APP/PS1 mice after BBB opening.
Conclusion
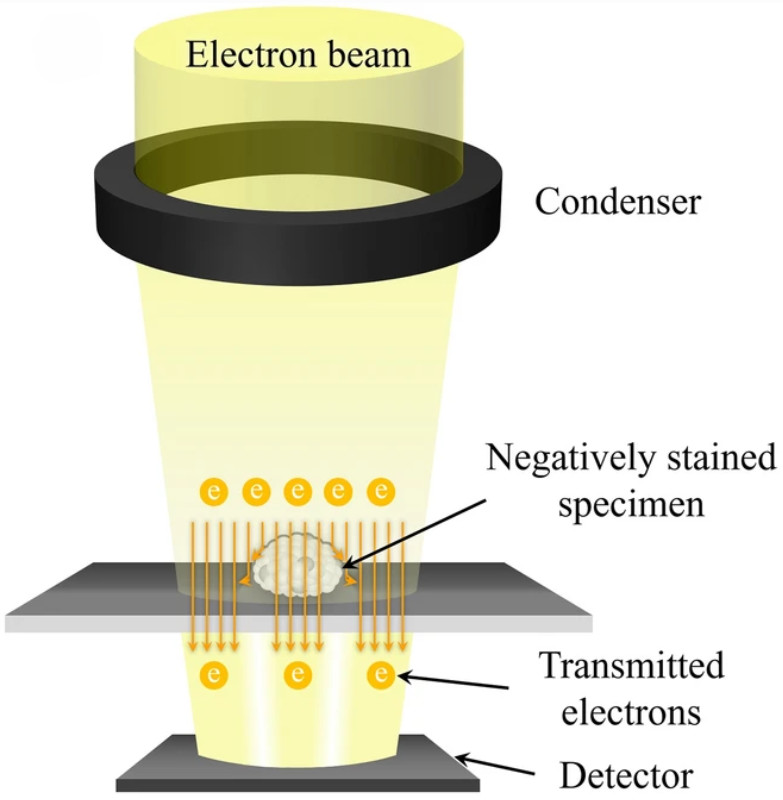
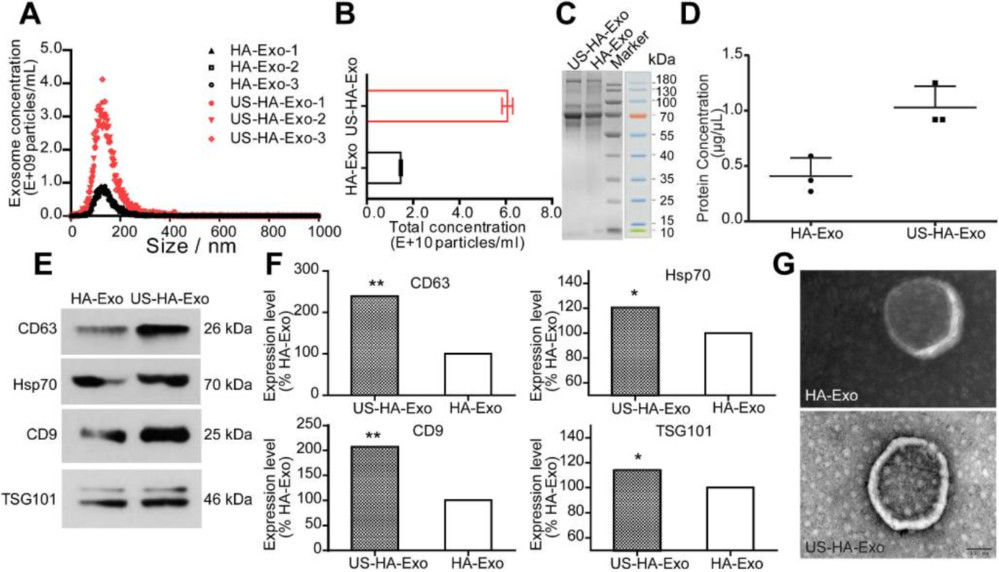 Figure 3. Characterization and Quantification of Astrocyte-Derived Exosomes. (A-B) NTA showing size distribution and total particle counts for exosomes from ultrasound-stimulated vs. untreated astrocytes. (C-D) Total protein assessment by SDS-PAGE and BCA. (E-F) Western blot of exosomal biomarkers comparing stimulated and control groups. (G) TEM image of exosome morphology (scale bar: 100 nm). (Deng Z, et al., 2021)
Figure 3. Characterization and Quantification of Astrocyte-Derived Exosomes. (A-B) NTA showing size distribution and total particle counts for exosomes from ultrasound-stimulated vs. untreated astrocytes. (C-D) Total protein assessment by SDS-PAGE and BCA. (E-F) Western blot of exosomal biomarkers comparing stimulated and control groups. (G) TEM image of exosome morphology (scale bar: 100 nm). (Deng Z, et al., 2021)
Precise exosome quantification enabled credible assessment of therapeutic efficacy. The case underscores why rigorous measurement is critical for validating vesicle-based interventions.
At Creative Biostructure, we recognize the challenges of precise exosome measurement. Our quantification services integrate particle counting, protein, lipid, and RNA analysis to generate reproducible and publication-ready results. From biomarker discovery to therapeutic development and manufacturing quality control, we provide reliable data and expert support. Contact us to discuss your project and explore how we can advance your research.
References
- Chia B S, Low Y P, Wang Q, et al. Advances in exosome quantification techniques. TrAC Trends in Analytical Chemistry. 2017, 86: 93-106.
- Deng Z, Wang J, Xiao Y, et al. Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity. Theranostics. 2021, 11(9): 4351.
- Zhang H, Wang S, Sun M, et al. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Frontiers in Immunology. 2023, 13: 1093607.
- Welsh J A, Goberdhan D C I, O'Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles. 2024, 13(2): e12404.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.