MSC and Stem Cell Derived Exosome Therapy Studies
Mesenchymal Stem Cells (MSCs) have long been the cornerstone of regenerative medicine, but live cell therapy faces significant hurdles: storage instability, immune rejection risks, and potential tumorigenicity. The industry is shifting toward the bioactive "secretome"—specifically, MSC Exosomes.
We provide comprehensive MSC and Stem Cell Exosome Therapy solutions. Whether you are sourcing exosomes from Bone Marrow, Adipose Tissue, or Umbilical Cord (Wharton's Jelly), we offer an integrated platform for isolation, characterization, and functional validation. We help you transform stem cell secretions into a consistent, scalable, and potent cell-free therapeutic product.
Challenges in MSC Exosome Development
While promising, translating MSC exosomes from bench to bedside requires overcoming significant manufacturing and quality control barriers.
- Donor Heterogeneity: Exosomes from different donors (or even different passages) can have vastly different cargoes. Standardizing the starting material is critical for consistent efficacy.
- Scalable Manufacturing: Producing clinical-grade quantities of exosomes requires moving from 2D flasks to 3D bioreactors without altering the vesicle's phenotype.
- Potency Definition: Regulatory agencies require distinct Mechanism of Action (MoA) data. Simply counting particles is not enough; you must prove biological activity (e.g., immunomodulation or angiogenesis).
- Purity Requirements: Separating therapeutic exosomes from cell debris and media contaminants (like bovine EVs) is essential to attribute efficacy solely to the drug product.
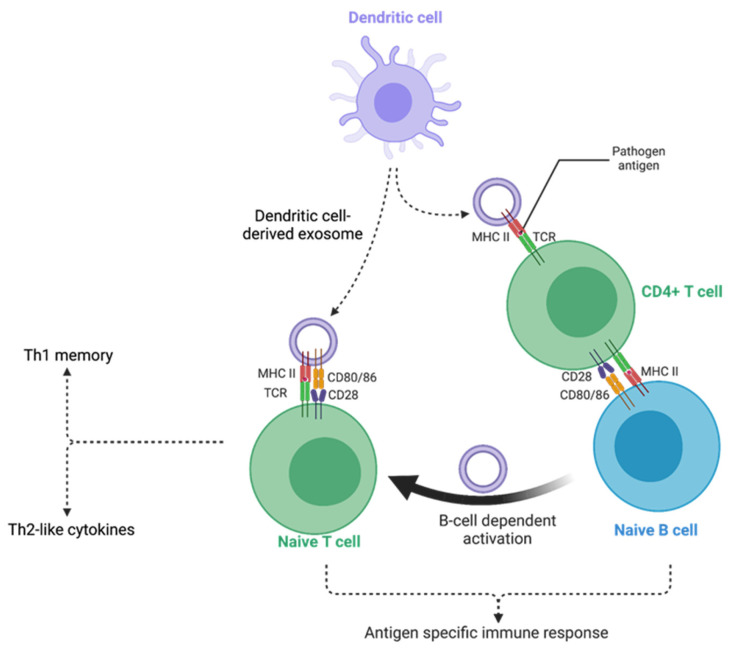
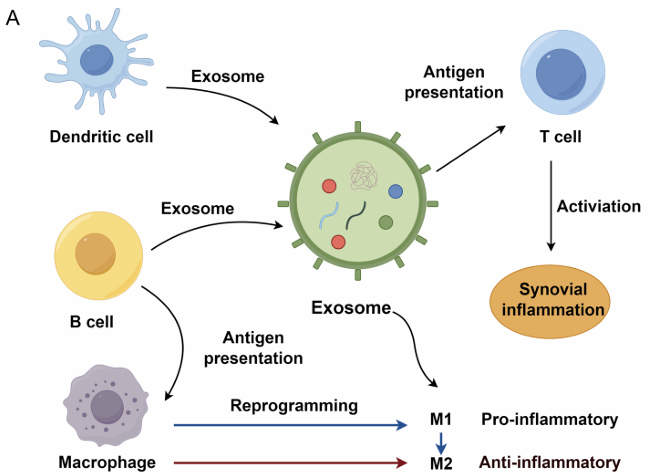
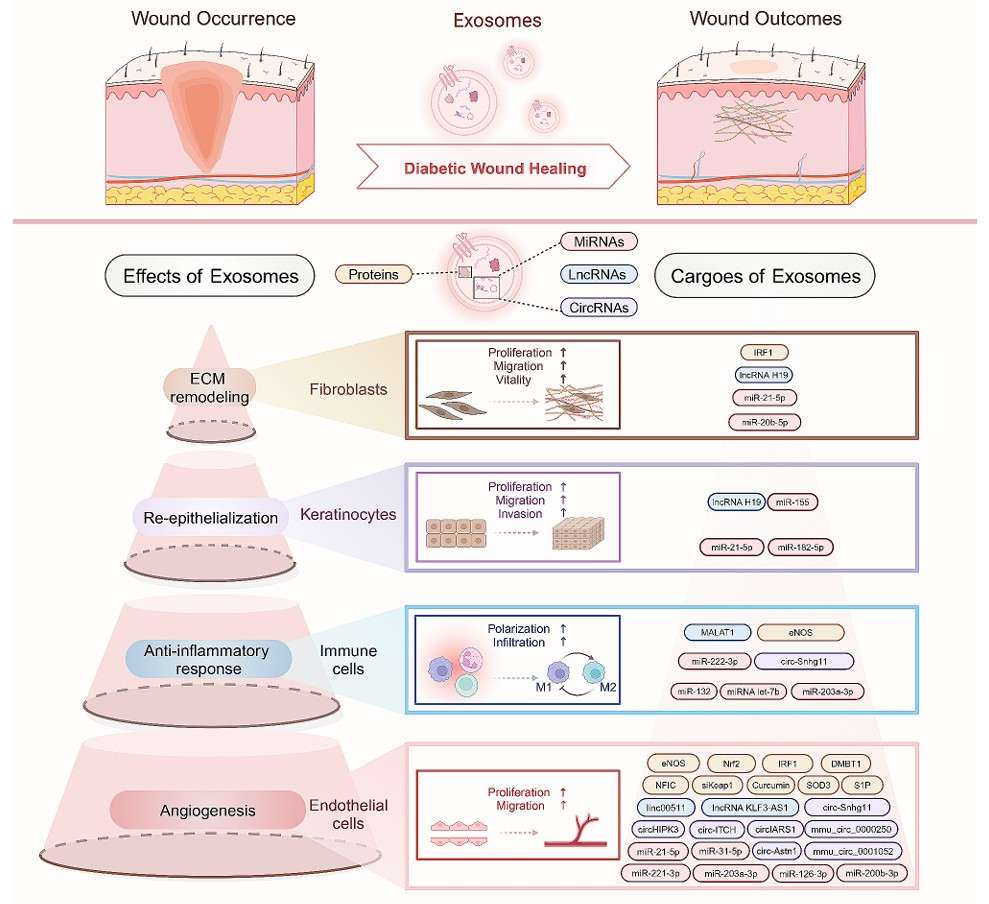
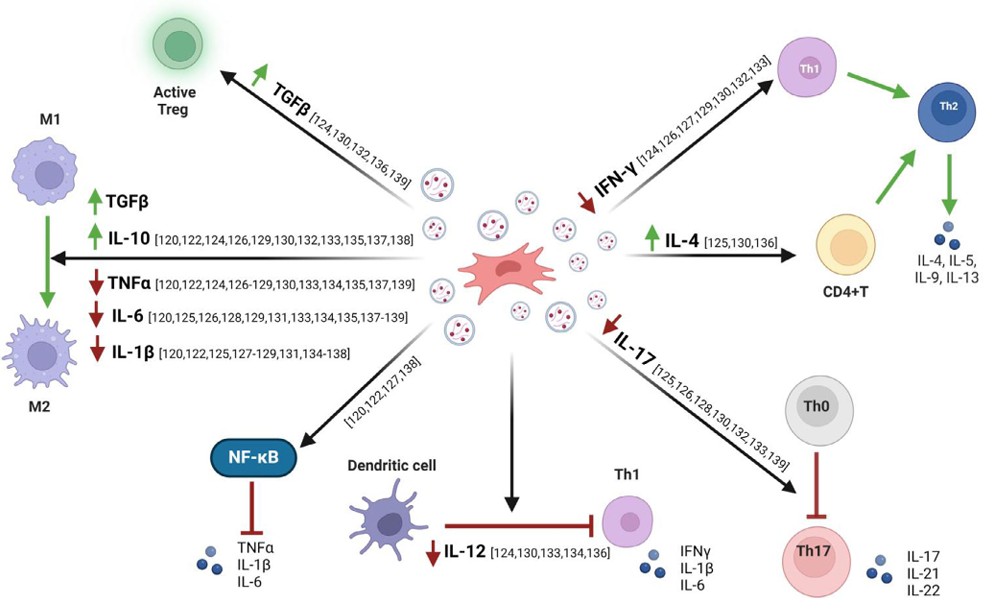 Figure 1. Mechanisms of mesenchymal stem cell-derived extracellular vesicles (EVs) in inflammatory bowel disease (IBD) therapy. (Clua-Ferré L, et al., 2024)
Figure 1. Mechanisms of mesenchymal stem cell-derived extracellular vesicles (EVs) in inflammatory bowel disease (IBD) therapy. (Clua-Ferré L, et al., 2024)
Our Therapeutic Development Workflow
We offer a modular pipeline designed to ensure the reproducibility and potency of your stem cell exosome product.
| Development Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Source Selection & Cell Banking | Optimized Cell Lines: We assist in selecting the optimal tissue source (e.g., Umbilical Cord vs. Adipose) based on your target indication. We establish Master Cell Banks (MCB) with full donor qualification to ensure traceability. | Exosome Isolation from Cell Culture Supernatants, Animal Tissue Exosome Isolation |
| Upstream Production | 3D Bioreactor Expansion: We utilize hollow-fiber or stirred-tank bioreactors to scale up MSC culture. We optimize media formulations (chemically defined, serum-free) to maximize exosome yield while minimizing contaminants. | Upstream Process Development (Cell Culture Optimization), |
| Downstream Purification | Scalable Isolation: We employ Tangential Flow Filtration (TFF) coupled with chromatography. This scalable workflow preserves vesicle integrity and removes proteins/aggregates more effectively than ultracentrifugation. | Downstream Purification & Formulation, Exosome Isolation by Tangential Flow Filtration (TFF) |
| Potency Validation | Functional Matrix: We validate bioactivity using a matrix of assays relevant to your MoA, such as T-cell suppression assays (for autoimmune disease) or Tube Formation assays (for vascular regeneration). | Immunomodulation and Inflammation Assays, Angiogenesis and Stem Cell Functional Assays |
Core Technologies for Regenerative Medicine
We deploy specific technologies to characterize the identity and function of stem cell exosomes.
Potency Assay Matrix
Defining Efficacy: Regulatory success depends on potency. We develop custom potency assays tailored to your therapeutic hypothesis. For wound healing, we use scratch migration and fibroblast proliferation assays. For immunomodulation, we measure the suppression of TNF-α/IFN-γ in activated PBMCs. These functional readouts serve as the lot-release criteria for your product.
Comprehensive Surface Profiling
Confirming Identity: We validate the identity of your MSC exosomes using the ISCT minimal criteria. We use Flow Cytometry and Western Blot to confirm the presence of canonical markers (CD73, CD90, CD105, CD63, CD81) and the absence of non-MSC markers (HL-DR, CD14), ensuring product purity and phenotype retention.
3D Culture & Preconditioning
Enhancing Potency: Standard 2D culture often yields low-potency exosomes. We utilize 3D spheroid culture or hypoxic preconditioning to mimic the in vivo niche. These stress signals trigger MSCs to secrete exosomes enriched in angiogenic factors (like VEGF) and anti-inflammatory miRNAs, significantly boosting therapeutic efficacy.
Application Spotlight: MSC Exosomes for Acute Kidney Injury Repair
This analysis demonstrates the regenerative potential of MSC exosomes in treating organ failure, validating their role as a cell-free alternative to stem cell transplantation.
Featured Technologies:
- MSC Exosome Isolation
- In Vivo Efficacy Testing
Literature Interpretation:
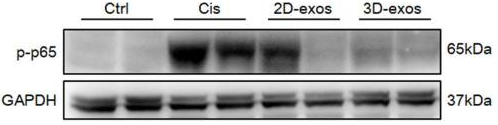 Figure 2. Western blot analysis of p-p65 expression in kidney tissues of cisplatin-treated mice, showing reduced levels after treatment with 3D-exos. (Cao J, et al., 2020)
Figure 2. Western blot analysis of p-p65 expression in kidney tissues of cisplatin-treated mice, showing reduced levels after treatment with 3D-exos. (Cao J, et al., 2020)
Acute Kidney Injury (AKI) is a severe condition with limited treatment options. Researchers investigated whether exosomes derived from human MSCs could repair renal damage. They isolated exosomes from MSCs and administered them to a cisplatin-induced AKI mouse model. The study showed that the MSC exosomes significantly improved renal function (reduced BUN/Creatinine) and reduced tubular cell apoptosis. Mechanistically, the exosomes delivered autophagy-regulating miRNAs that protected kidney cells from oxidative stress. This outcome confirms that MSC-derived exosomes possess intrinsic tissue-repair capabilities comparable to their parent cells, validating approach of developing cell-free formulations for complex organ regeneration.
Start Your Therapeutic Project
We make getting started straightforward. Our process is designed to be collaborative and transparent.
How It Works: Our Project Pathway
 Figure 3. Our end-to-end workflow for developing consistent and potent cell-free therapeutics from Mesenchymal Stem Cells. (Creative Biostructure)
Figure 3. Our end-to-end workflow for developing consistent and potent cell-free therapeutics from Mesenchymal Stem Cells. (Creative Biostructure)
Ready to develop a scalable cell-free therapy? Our scientific team is available for a free consultation to discuss your MSC exosome development strategy. Contact us today to discuss your project.
References
- Clua-Ferré L, Suau R, Vañó-Segarra I, et al. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease. Clin Transl Med. 2024 Nov;14(11):e70075.
- Cao J, Wang B, Tang T, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020 May 27;11(1):206.