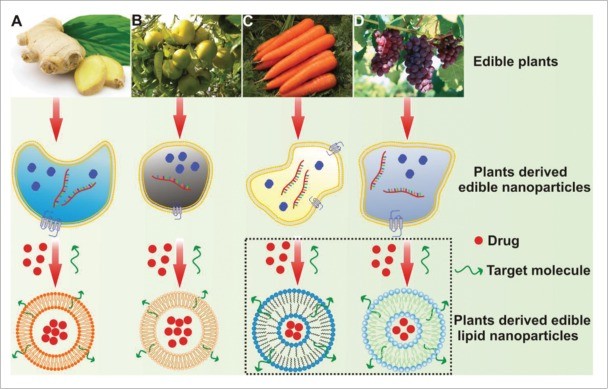
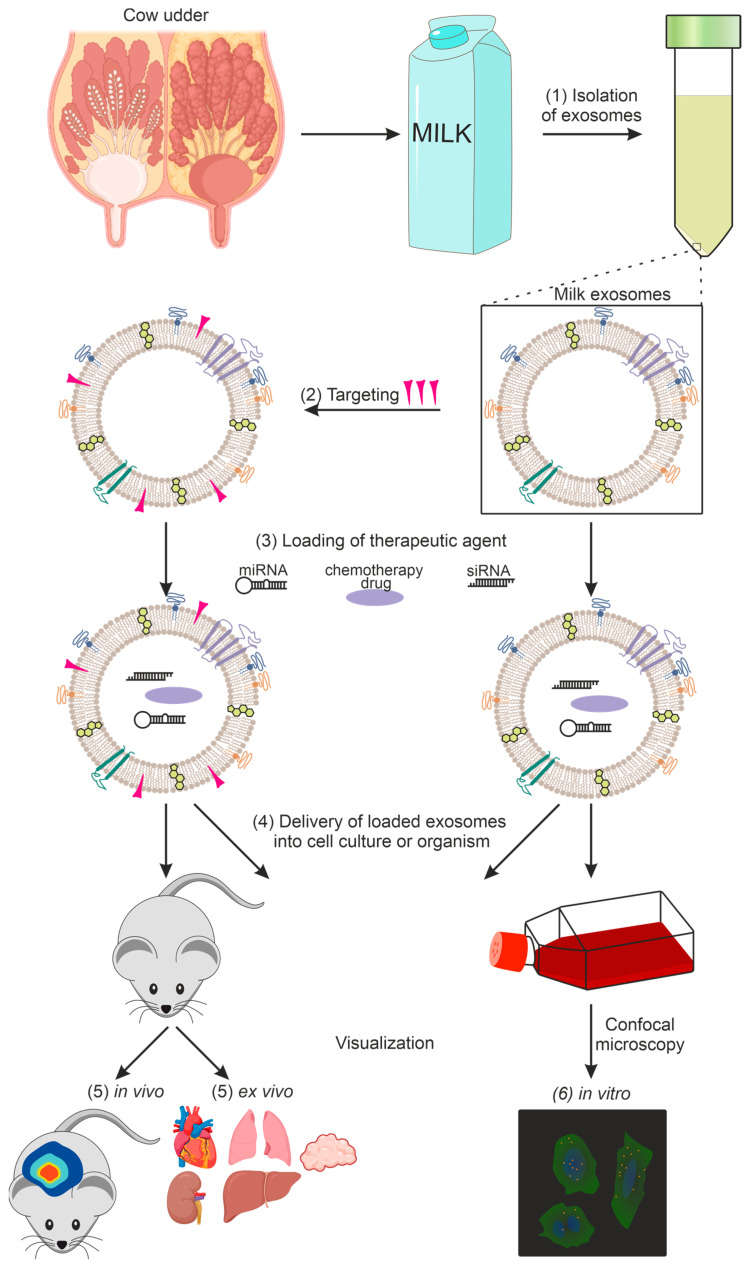
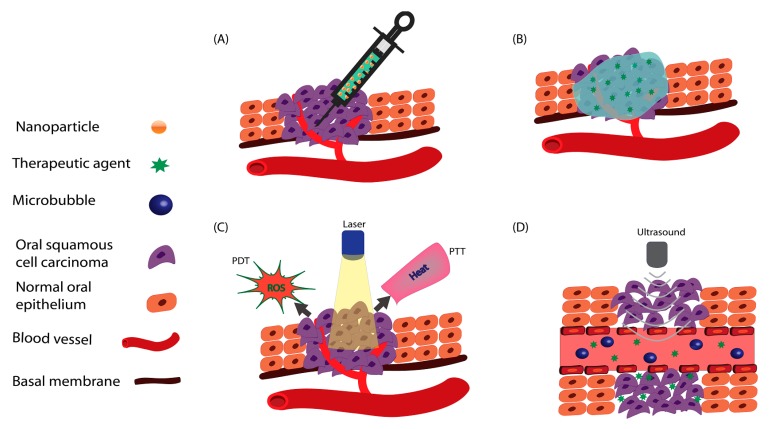
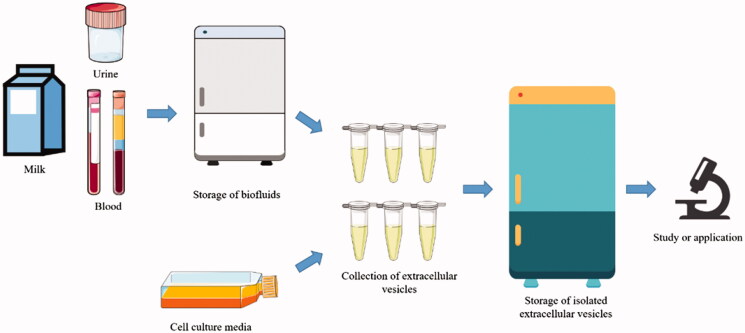
Gastrointestinal Functional Evaluation of Exosomes
For oral exosome therapeutics and functional foods, surviving the stomach is just the first hurdle. True efficacy depends on what happens next: uptake by intestinal epithelial cells, transport across the mucosal barrier, and functional activity in the target tissue.
We provide comprehensive Gastrointestinal Functional Evaluation services. Moving beyond simple stability tests, we offer an integrated platform to assess the bioaccessibility, bioavailability, and biological function of your exosomes in the GI tract. From digestion models to functional assays, we help you prove that your product not only survives digestion but delivers measurable health benefits.
Evaluating Function in the GI Environment
A robust evaluation strategy answers three critical questions for regulatory and R&D purposes.
- Digestive Stability (Bioaccessibility): Can the exosomes resist the harsh pH 2.0 of the stomach and the bile salts of the intestine? We quantify the fraction of exosomes that remain structurally intact and cargo-loaded after digestion.
- Intestinal Uptake (Bioavailability): Do the surviving exosomes actually enter the gut cells? We evaluate cellular uptake efficiency and transport mechanisms (e.g., endocytosis vs. paracellular) using polarized cell models.
- Biological Activity (Function): Once absorbed, do they work? We test if the digested exosomes can still exert their intended effect—such as suppressing inflammation (anti-colitis) or protecting the gut barrier (tight junction integrity).
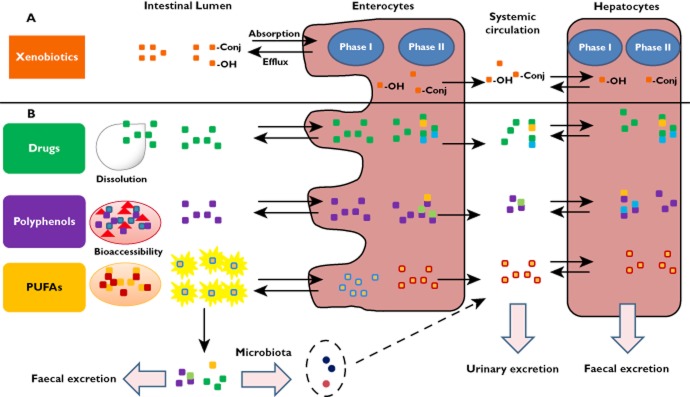 Figure 1. Overview of xenobiotic absorption, metabolism, and excretion (A), with examples of compounds such as drugs, polyphenols, and PUFAs (B). (Rein MJ, et al., 2013)
Figure 1. Overview of xenobiotic absorption, metabolism, and excretion (A), with examples of compounds such as drugs, polyphenols, and PUFAs (B). (Rein MJ, et al., 2013)
Our Functional Evaluation Workflow
We simulate the complete journey from ingestion to action using standardized physiological models.
| Evaluation Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Standardized Digestion | Membrane Integrity Check: Beyond simple counting, we verify chemical stability. We use Lipidomics to detect if pancreatic lipases have hydrolyzed the exosome membrane lipids (e.g., Lyso-PC formation), providing molecular proof of resistance. | Human Exosome Lipidomics Analysis |
| Mucus Interaction | Diffusion Tracking: To test mucus penetration, we use Confocal Microscopy (CLSM) on mucin-coated slides. We also measure Zeta Potential changes to predict electrostatic repulsion/adhesion forces with mucin. | Exosome Characterization by NTA |
| Cellular Uptake (Bioavailability) | Quantifying Absorption: We don't just guess; we count. We use Nano-Flow Cytometry (nFCM) to precisely quantify the percentage of Caco-2 cells that have internalized the exosomes after digestion exposure. | Exosome Characterization by NanoFCM |
| Functional Readout | Barrier Mechanism: To prove gut health benefits, we go to the gene level. We use RT-qPCR to measure the upregulation of Tight Junction genes (ZO-1, Claudin-1) in the barrier model, proving the molecular mechanism of repair. | Exosomal mRNA Sequencing |
Core Technologies for Functional Assessment
We utilize advanced assays to link physical stability with biological function.
Post-Digestion Cargo Integrity
Is the Payload Safe? Structural survival doesn't guarantee cargo protection. After simulated digestion, we extract RNA or proteins from the exosomes and use qPCR or Western Blot to verify that the therapeutic molecules (e.g., miRNA-21, Curcumin) are not degraded, ensuring the functional component is still active.
Intestinal Barrier Repair Assays
Proving Gut Health Benefits: Many oral exosomes target Leaky Gut. We treat compromised Caco-2 monolayers (induced by DSS or TNF-alpha) with your exosomes. We then measure the restoration of TEER values and the upregulation of tight junction proteins (ZO-1, Occludin) to validate barrier repair efficacy.
Immunomodulation Screens
Gut Immunity: The gut is the largest immune organ. We use co-cultures of intestinal epithelial cells and immune cells (PBMCs/Macrophages). We measure whether your exosomes can shift the immune response from pro-inflammatory (IL-6, IL-1beta) to tolerogenic (IL-10, TGF-beta) phenotypes, a key claim for functional foods.
Application Spotlight: Milk Exosomes Protect Fragile Cargo During Digestion
This analysis highlights the innate stability of milk exosomes and their ability to shield hydrophobic nutrients like curcumin from digestive degradation.
Featured Technologies:
- Simulated Gastrointestinal Digestion
- Caco-2 Permeability Assay
Literature Interpretation:
Curcumin is a potent anti-inflammatory agent but has poor stability and bioavailability. It degrades rapidly in the gut. Researchers encapsulated curcumin into milk exosomes and subjected them to simulated gastric (pepsin, pH 2) and pancreatic digestion. While free curcumin degraded significantly, the milk exosome-encapsulated curcumin remained stable throughout the digestive process. Furthermore, the exosome formulation showed significantly higher permeability across Caco-2 monolayers compared to free drug. This study confirms that milk exosomes act as a robust "digestive shield," protecting sensitive cargos and enhancing their intestinal uptake.
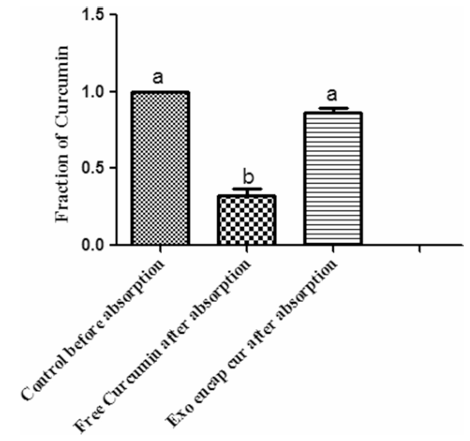 Figure 2. Exosome-mediated enhancement of curcumin transport across Caco-2 intestinal epithelial cells. (Vashisht M, et al., 2017)
Figure 2. Exosome-mediated enhancement of curcumin transport across Caco-2 intestinal epithelial cells. (Vashisht M, et al., 2017)
Start Your Evaluation Project
Don't launch a product that fails in the stomach. Validate your formulation with the industry-standard digestion model.
How It Works: Our Project Pathway
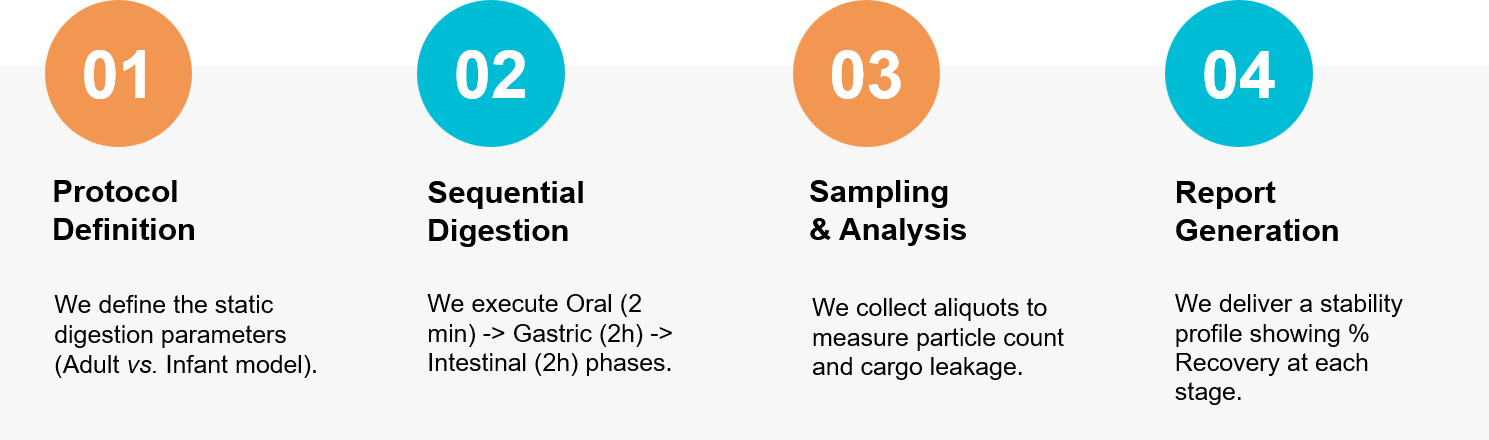 Figure 3. Our integrated workflow for evaluating the bioaccessibility and functional bioactivity of exosomes in the human gastrointestinal tract. (Creative Biostructure)
Figure 3. Our integrated workflow for evaluating the bioaccessibility and functional bioactivity of exosomes in the human gastrointestinal tract. (Creative Biostructure)
Ready to evaluate the true potential of your oral exosomes? Our food scientists are available to design a custom functional evaluation study. Contact us today to discuss your project.
References
- Rein MJ, Renouf M, Cruz-Hernandez C, et al. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol. 2013 Mar;75(3):588-602.
- Vashisht M, Rani P, Onteru SK, et al. Curcumin Encapsulated in Milk Exosomes Resists Human Digestion and Possesses Enhanced Intestinal Permeability in Vitro. Appl Biochem Biotechnol. 2017 Nov;183(3):993-1007.