Nano-Flow Cytometry (nFCM)-Based Exosome Characterization Service
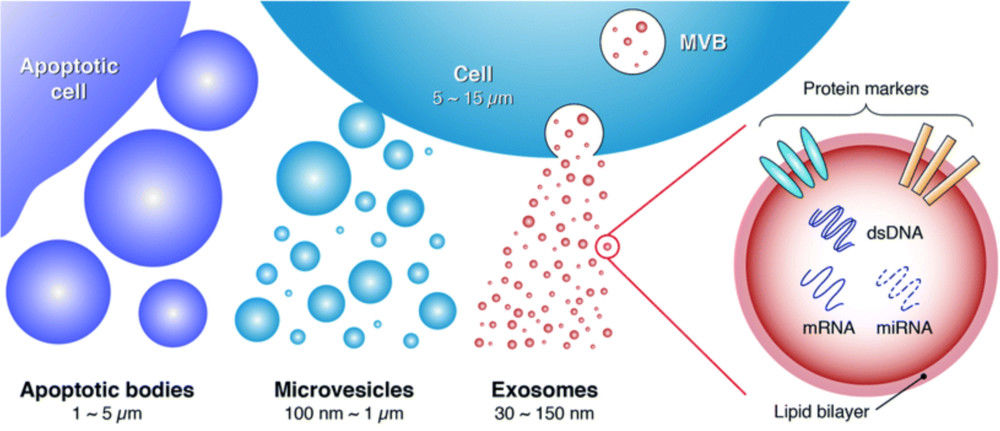
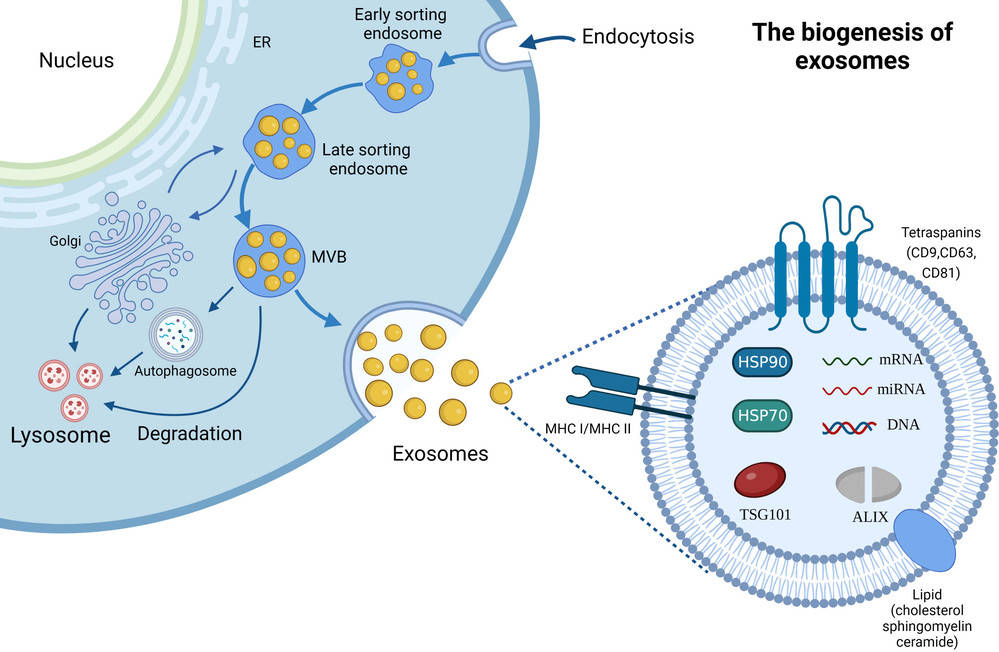
Exosomes are nanoscale extracellular vesicles (EVs) secreted by almost all cell types, mediating intercellular communication by transferring proteins, lipids, and nucleic acids. Their central roles in disease mechanisms, biomarker discovery, and therapeutic development make accurate and reproducible characterization essential.
Traditional bulk methods such as immunoblotting and ELISA provide useful information but often lack the ability to resolve exosome heterogeneity at the single-vesicle level. To overcome these limitations, Creative Biostructure offers a dedicated Nano-Flow Cytometry (nFCM) service, enabling high-resolution single-vesicle characterization of exosomes in compliance with MISEV2023 guidelines and the MIFlowCyt-EV reporting framework.
What is Nano-Flow Cytometry?
Nano-Flow Cytometry (nFCM) is an advanced, high-sensitivity flow cytometry platform specifically optimized for the analysis of nanoscale particles such as extracellular vesicles. Unlike conventional flow cytometry designed for cells, nFCM employs an enhanced optical system capable of detecting extremely weak light scattering and fluorescence signals from particles as small as ~40 nm.
This technology enables precise, multi-parametric single-vesicle analysis, providing key insights into:
- Size Distribution: Accurate measurement of particle size and population heterogeneity.
- Absolute Concentration: Direct quantification of vesicle counts without reliance on relative estimates.
- Phenotypic Profiling: Detection of surface markers and cargo using fluorescent labeling, allowing identification of distinct subpopulations.
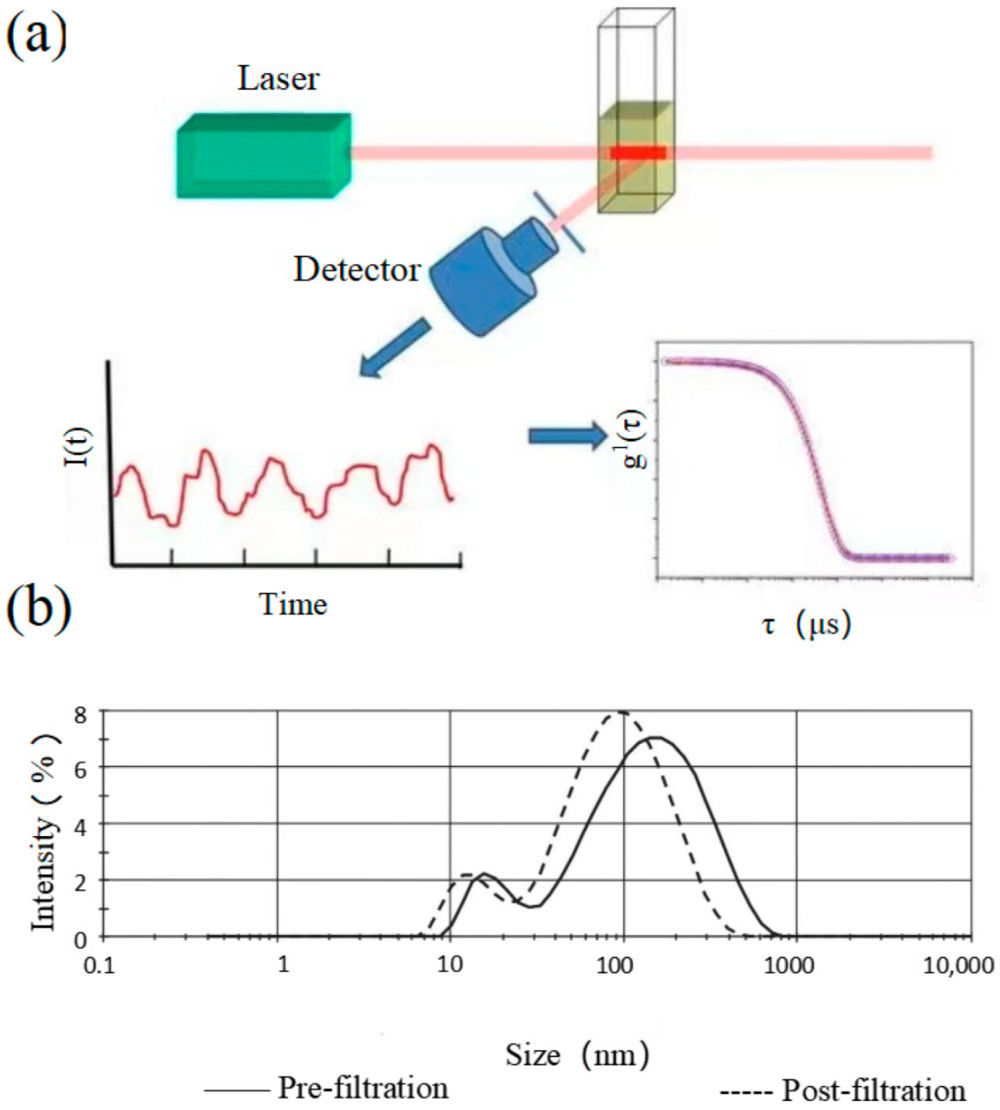
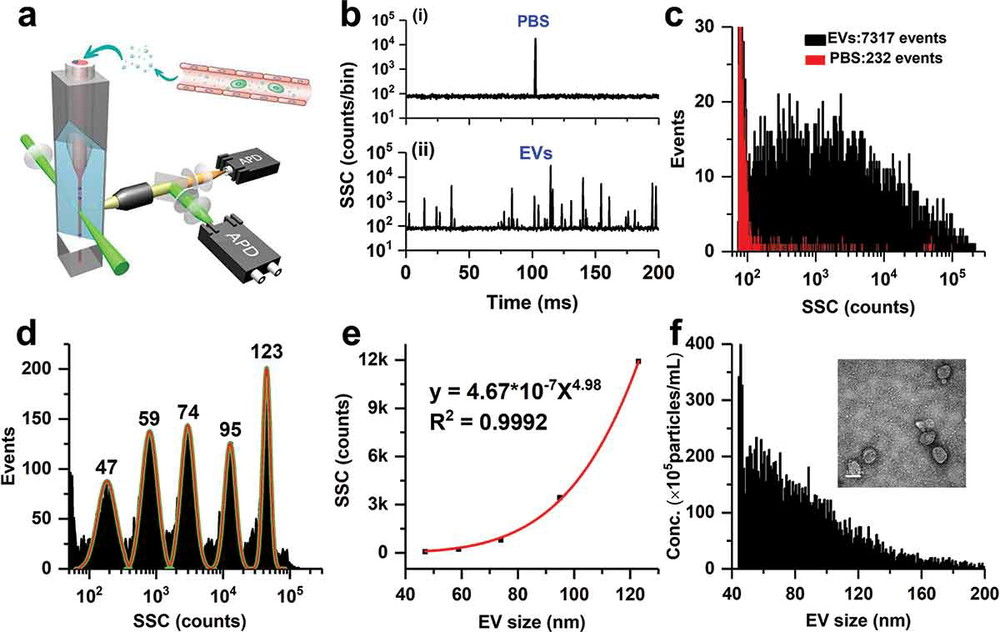 Figure 1. Measurement of EV particle size and concentration using nano-flow cytometry (nFCM). (a) Schematic diagram of the laboratory-built nFCM system. (b) Representative side scatter (SSC) burst traces of PBS (i) and EVs isolated from platelet-free plasma (PFP) by ultracentrifugation (UC) (ii). (c) SSC distribution histograms for PBS (red, 232 events) and EVs (black, 7317 events) collected over 2 min. (d) SSC burst area histogram of five monodisperse silica nanoparticles (SiNPs; 47-123 nm) fitted with Gaussian curves. (e) Calibration curve plotting Gaussian-fitted SSC intensity (after refractive index correction) versus particle size. (f) Size distribution histogram of EVs (n = 7317, bin width = 1 nm), with representative TEM micrograph inset (scale bar: 100 nm). (Tian Y, et al., 2020)
Figure 1. Measurement of EV particle size and concentration using nano-flow cytometry (nFCM). (a) Schematic diagram of the laboratory-built nFCM system. (b) Representative side scatter (SSC) burst traces of PBS (i) and EVs isolated from platelet-free plasma (PFP) by ultracentrifugation (UC) (ii). (c) SSC distribution histograms for PBS (red, 232 events) and EVs (black, 7317 events) collected over 2 min. (d) SSC burst area histogram of five monodisperse silica nanoparticles (SiNPs; 47-123 nm) fitted with Gaussian curves. (e) Calibration curve plotting Gaussian-fitted SSC intensity (after refractive index correction) versus particle size. (f) Size distribution histogram of EVs (n = 7317, bin width = 1 nm), with representative TEM micrograph inset (scale bar: 100 nm). (Tian Y, et al., 2020)
Why Choose nFCM for Exosome Characterization?
Nano-Flow Cytometry is increasingly recognized as a gold-standard method for exosome analysis, offering greater accuracy and depth than bulk or imaging-based techniques. Its main advantages include:
- Superior resolution and sensitivity: Detects single vesicles down to ~40 nm, including populations missed by nanoparticle tracking analysis (NTA) or conventional cytometry.
- Multiparametric analysis: Measures size, concentration, and multiple fluorescent markers in one assay, capturing vesicle heterogeneity.
- High throughput with reproducibility: Processes thousands of particles per second with consistent calibration, ensuring reliable results.
- Absolute quantification: Provides true particle counts rather than relative estimates, enabling accurate comparisons across studies.
With these strengths, nFCM delivers comprehensive insights for biomarker discovery, therapeutic EV quality control, and translational research.
Our NanoFCM Exosome Characterization Service
At Creative Biostructure, we offer end-to-end nFCM exosome characterization, combining advanced instrumentation with strict quality standards. Our platform provides single-vesicle resolution with calibrated measurements of size, concentration, and surface markers, delivering reproducible, publication-ready results.
Step-by-Step Workflow of Our nFCM Exosome Characterization
Project Consultation
Initial consultation to understand research objectives, sample type, and analytical requirements, followed by a customized study design tailored to client goals.
Sample Submission & Exosome Isolation
Clients may submit biofluids, cell culture supernatants, or pre-isolated exosomes, with optional exosome isolation by ultracentrifugation, size exclusion chromatography (SEC), or tangential flow filtration (TFF) to ensure high-quality vesicles.
Sample Preparation
Exosome samples are prepared for analysis and, when needed, labeled with fluorescent antibodies or dyes targeting surface proteins or internal cargo.
Instrument Calibration
Standard nanoparticles and fluorescence reference beads are used to calibrate light scatter, fluorescence intensity, and volume measurements, ensuring reproducible and quantitative results.
NanoFCM Data Acquisition
Single-vesicle analysis is performed to capture size distribution, absolute concentration, and surface marker expression, revealing vesicle heterogeneity at high resolution.
Data Analysis & Interpretation
Raw data are processed into comprehensive results, including graphs, statistical comparisons, and phenotypic profiles of exosome subpopulations.
Report Delivery
A publication-ready report is provided, containing raw data, processed results, and expert interpretation, ensuring transparency and reproducibility.
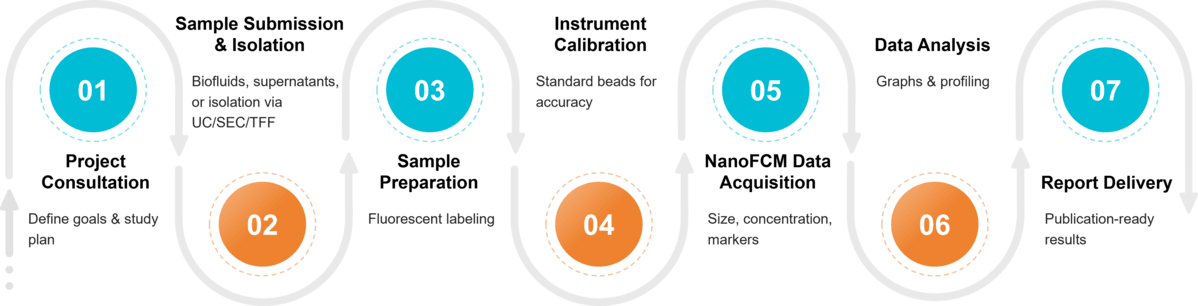 Figure 2. Project Workflow for Exosome Analysis by Nano-flow Cytometry. (Creative Biostructure)
Figure 2. Project Workflow for Exosome Analysis by Nano-flow Cytometry. (Creative Biostructure)
Sample Requirements for nFCM Assay
To ensure optimal results, we recommend:
| Parameter | Requirement |
|---|---|
| Volume | ≥ 20 μL per assay |
| Concentration | ≥ 1 × 1010 particles/mL (by NTA) or ≥ 5 μg per assay (by BCA) |
| Sample Types | Plasma, serum, urine, cerebrospinal fluid, cell culture supernatant, or purified EVs |
| Preservation | Fresh or frozen at -80 °C; avoid repeated freeze-thaw cycles |
What Deliverables Will You Receive
Clients receive a comprehensive data package including:
- Size distribution histograms and concentration curves
- Quantitative marker expression analysis (CD9, CD63, CD81, or custom antibodies)
- Epitope density reports and fluorescence calibration data
- Detailed experimental parameters in alignment with MIFlowCyt-EV standards
- Interpretive summary to support publication or regulatory submission
Applications of nFCM in Exosome Research
- Biomarker discovery in cancer, neurodegeneration, and infectious diseases
- Quality control for therapeutic EVs and exosome-based drug delivery
- Functional characterization of engineered or modified exosomes
- Comparative studies of exosome populations under different biological or experimental conditions
Comparison with Alternative Techniques
| Technique | Resolution | Parameters Measured | Throughput | Sorting | Best Suited For |
|---|---|---|---|---|---|
| NanoFCM | ~40 nm | Size, concentration, surface markers | High | No | Biomarker discovery, therapeutic QC |
| FACS (Single-EV Flow Cytometry) | ~100 nm (depending on instrument) | Multiplex surface marker profiling, subpopulation typing | Medium-High | Yes (for larger EVs) | Subpopulation classification, biomarker validation |
| NTA (Nanoparticle Tracking) | ~70 nm | Size & concentration only | Medium | No | General EV sizing |
| TEM (Transmission Electron Microscopy) / AFM (Atomic Force Microscopy) | ~1-2 nm | Morphology only | Low | No | Structural validation |
| Bead-based Flow Cytometry | >100 nm | Bulk surface marker detection (semi-quantitative) | High | No | Confirmatory marker analysis of surface proteins |
Key Features of Our Service
- High sensitivity: Single-vesicle detection down to ~40 nm with low background fluorescence.
- Comprehensive analysis: Quantitative measurement of size, concentration, and surface markers.
- Reproducibility: Standardized calibration ensures reliable, high-throughput data acquisition.
- Compliance: Fully aligned with MISEV2023 and the MIFlowCyt-EV framework for publication-ready results.
- Expertise & Trust: Experienced scientific team, proven track record with academic and industry partners, and rigorous quality management.
Case Study
Case: CD63 Characterization of Exosomes Using Nano-Flow Cytometry
Objective
To assess CD63 expression in four exosome samples derived from cerebrospinal fluid (CSF) and serum.
Methods
- Samples: CSF exosome 1 and Serum exosome 1 (Creative Biostructure); Plasma exosome and CSF exosome 2 (client).
- Preparation: Exosomes incubated with fluorescent CD63 antibody; unbound antibodies removed by ultracentrifugation.
- Analysis: Measured on Flow NanoAnalyzer using 488 nm laser; size and fluorescence recorded and analyzed with NanoFCM Professional Suite.
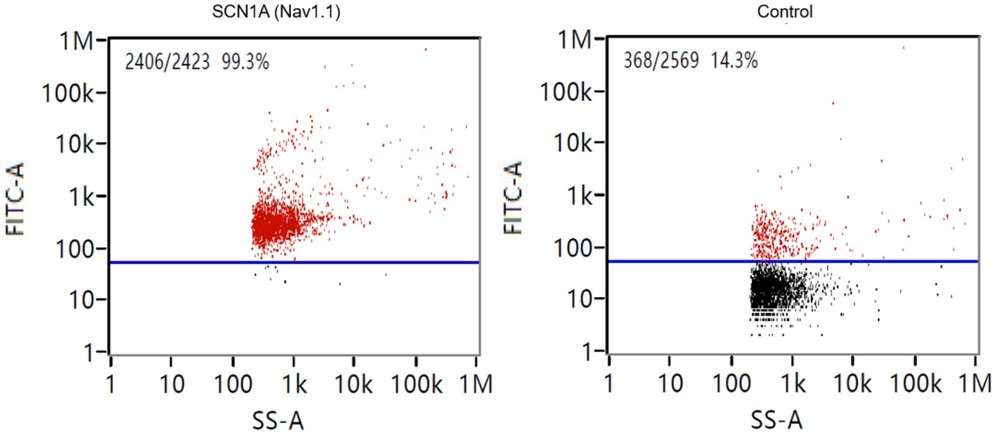 Figure 3. Nano-flow cytometry result for CSF exosome 1 sample characterized with CD63. (Creative Biostructure)
Figure 3. Nano-flow cytometry result for CSF exosome 1 sample characterized with CD63. (Creative Biostructure)
Results
- CSF exosome 1 and Serum exosome 1 showed detectable CD63 signals, higher than controls.
- Expression levels were low but measurable, confirming vesicle heterogeneity.
- Non-specific antibody binding was minimal, and background noise was effectively reduced.
Contribution of Creative Biostructure
- Supplied high-quality CSF and serum exosome samples.
- Performed labeling, ultracentrifugation, and NanoFCM assays.
- Delivered reproducible data and clear marker profiles.
Conclusion
This study demonstrated the value of NanoFCM for exosome marker detection, enabling sensitive, single-vesicle analysis that traditional cytometry could not achieve. Creative Biostructure's expertise ensured accurate results and reliable data interpretation.
At Creative Biostructure, we combine advanced Nano-Flow Cytometry (NanoFCM) with traditional flow cytometry-based assays to deliver reliable and reproducible exosome characterization. Whether you need fundamental marker profiling or high-resolution single-vesicle analysis, our team provides data you can trust for both research and downstream applications. Contact us to discuss your project and receive a customized solution.
References
- Tian Y, Gong M, Hu Y, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. Journal of Extracellular Vesicles. 2020, 9(1): 1697028.
- Welsh J A, Goberdhan D C I, O'Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles. 2024, 13(2): e12404.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.