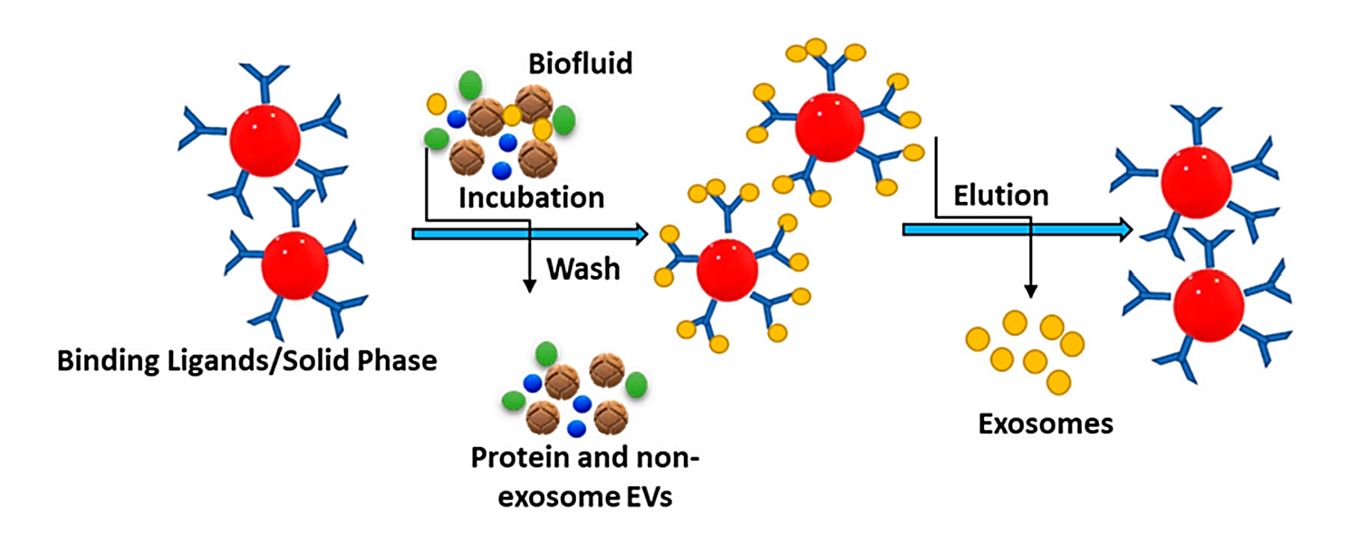
Size Exclusion Chromatography (SEC)-Based Exosome Purification Service
Isolating exosomes with high purity and structural integrity is essential for downstream success. At Creative Biostructure, we offer Size Exclusion Chromatography (SEC)-based exosome purification services designed to gently separate intact, functional exosomes from complex biological matrices with minimal contamination. SEC is a powerful technique that preserves vesicle integrity while achieving superior purity, making it an ideal choice for applications requiring functionally active and reproducible exosome preparations.
What Is Size Exclusion Chromatography for Exosomes?
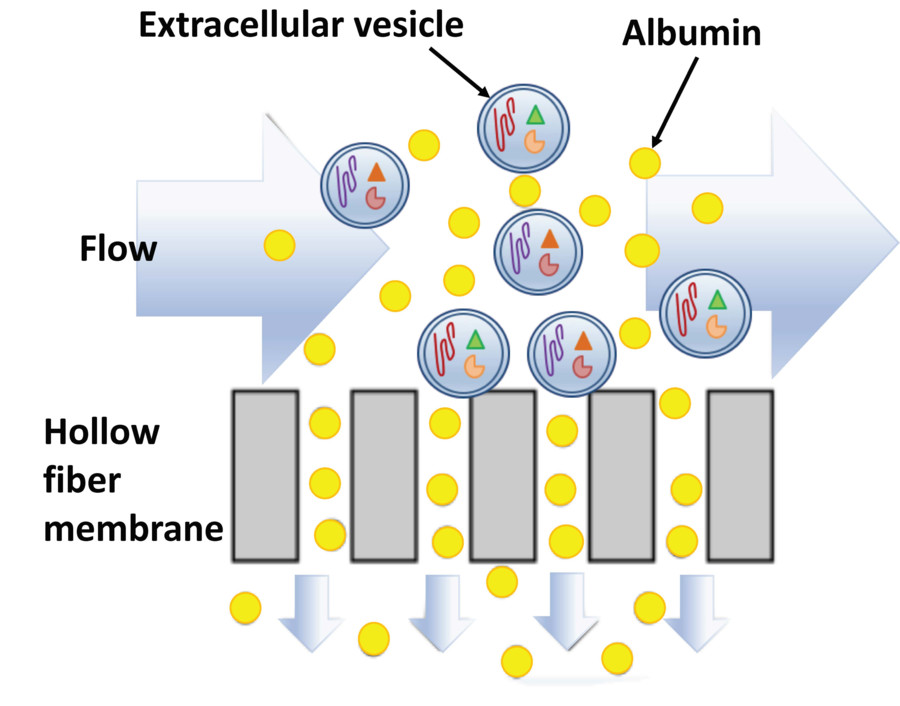
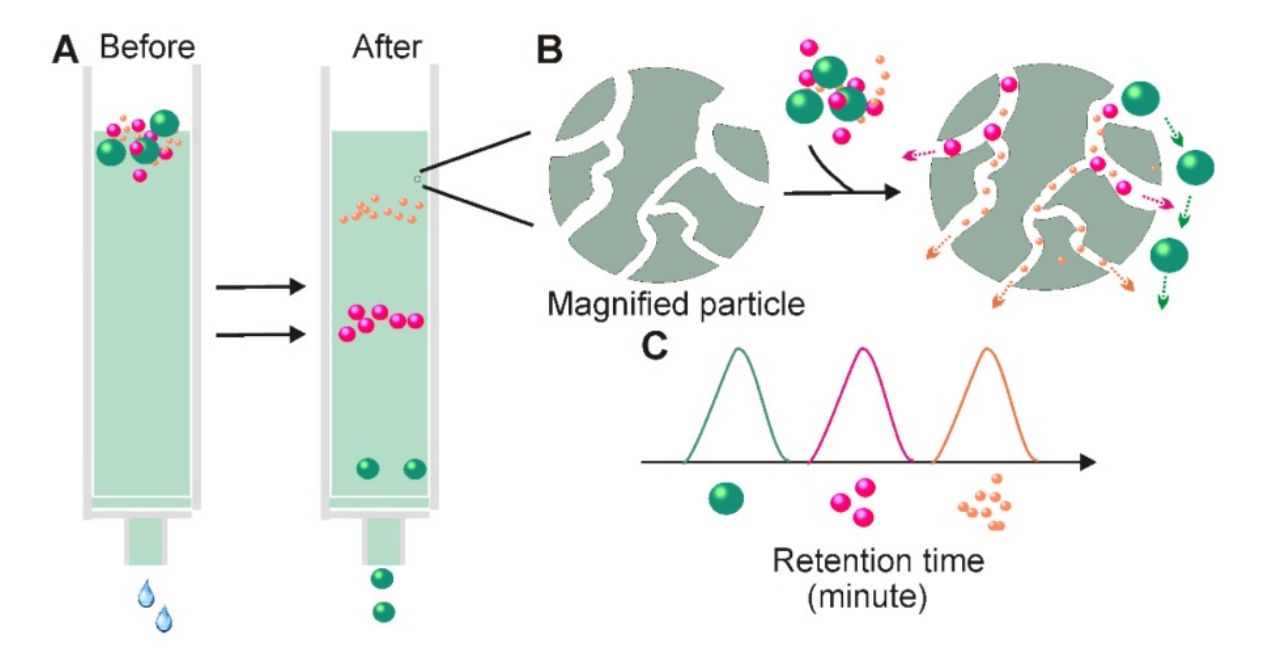
Size Exclusion Chromatography (also known as gel filtration or molecular sieve chromatography) separates molecules based on hydrodynamic size. As a sample passes through a column packed with porous beads, smaller molecules penetrate the pores and elute later, while larger particles like exosomes are excluded and elute earlier.
Unlike harsh methods such as ultracentrifugation, SEC applies no external force, allowing for the gentle and reliable isolation of extracellular vesicles while minimizing protein and lipoprotein contamination.
Why Choose SEC for Exosome Purification?
- High Purity: Efficiently separates exosomes from proteins and other small contaminants, enhancing the particle-to-protein ratio.
- Preserved Bioactivity: Maintains vesicle morphology and biological function due to minimal shear or mechanical stress.
- Scalability: Suitable for a range of sample volumes (from µL to 100 mL) and sources including plasma, serum, urine, and cell culture media.
- Reproducibility: Offers consistent results across batches and users when combined with automated fraction collection.
- Downstream Compatibility: Ideal for omics profiling (RNA-seq, proteomics), therapeutic development, and biodistribution studies.
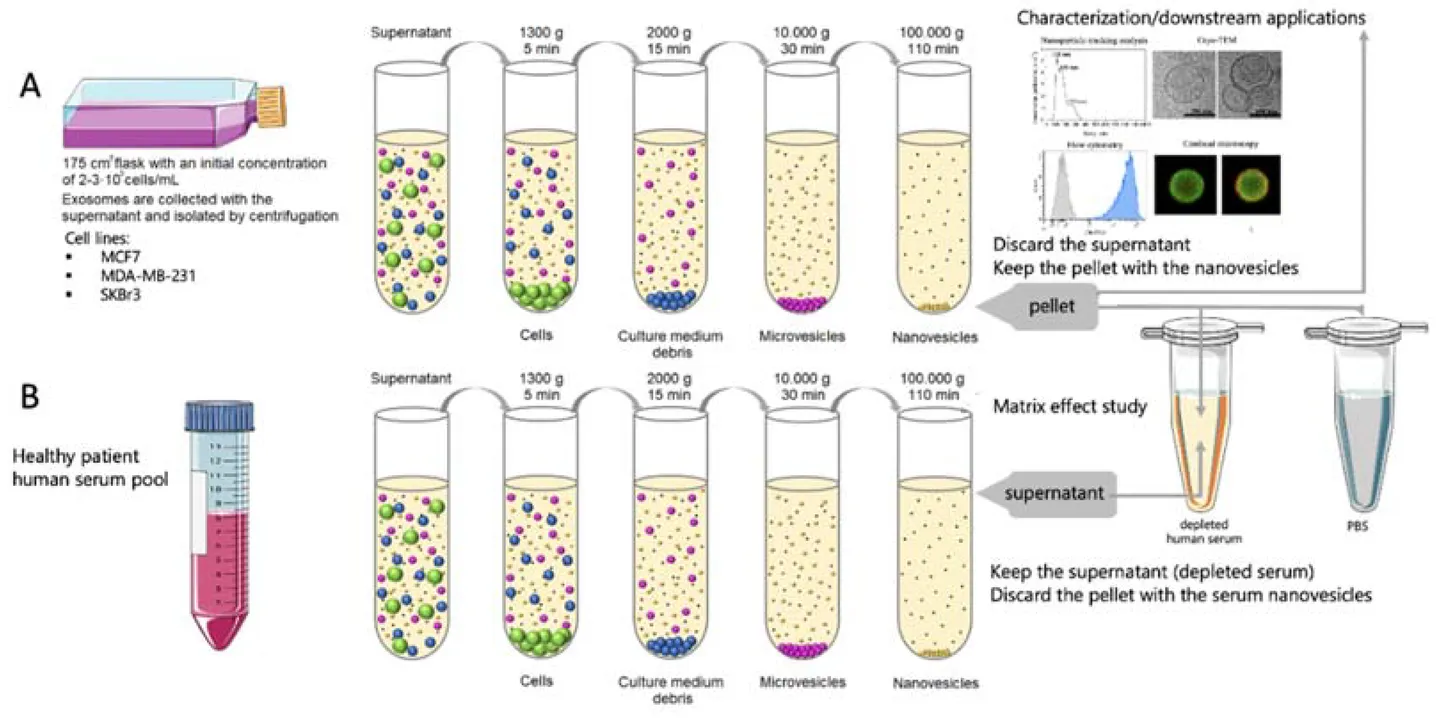
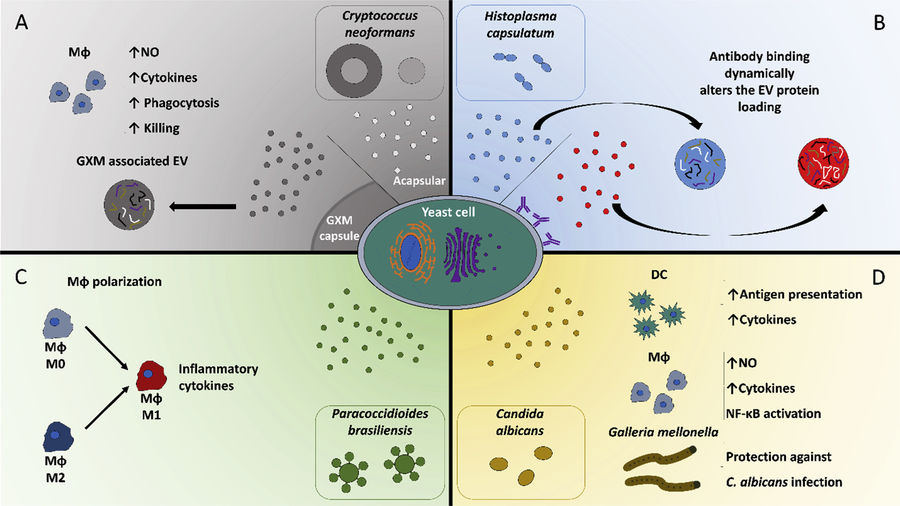
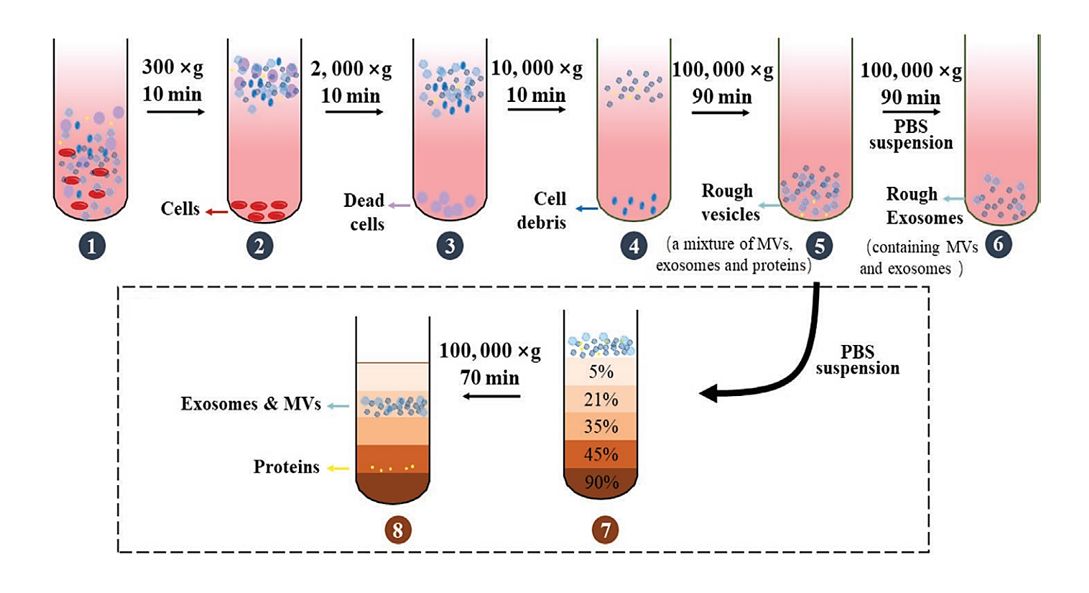
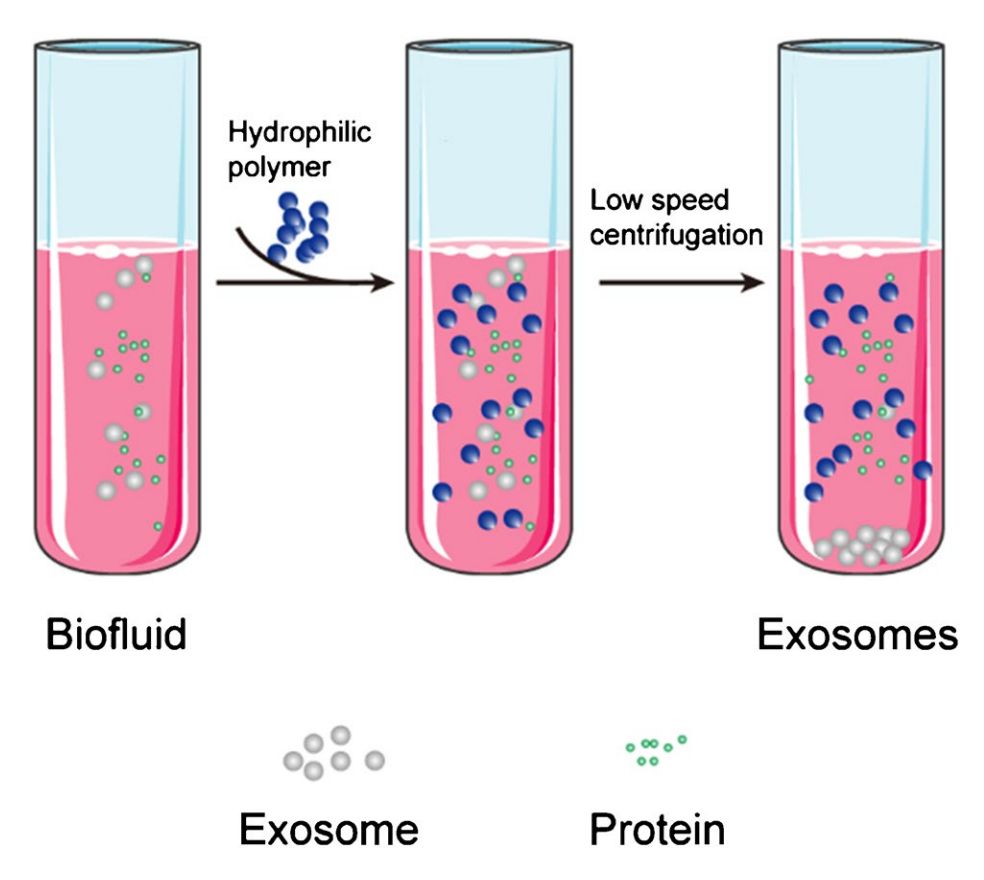
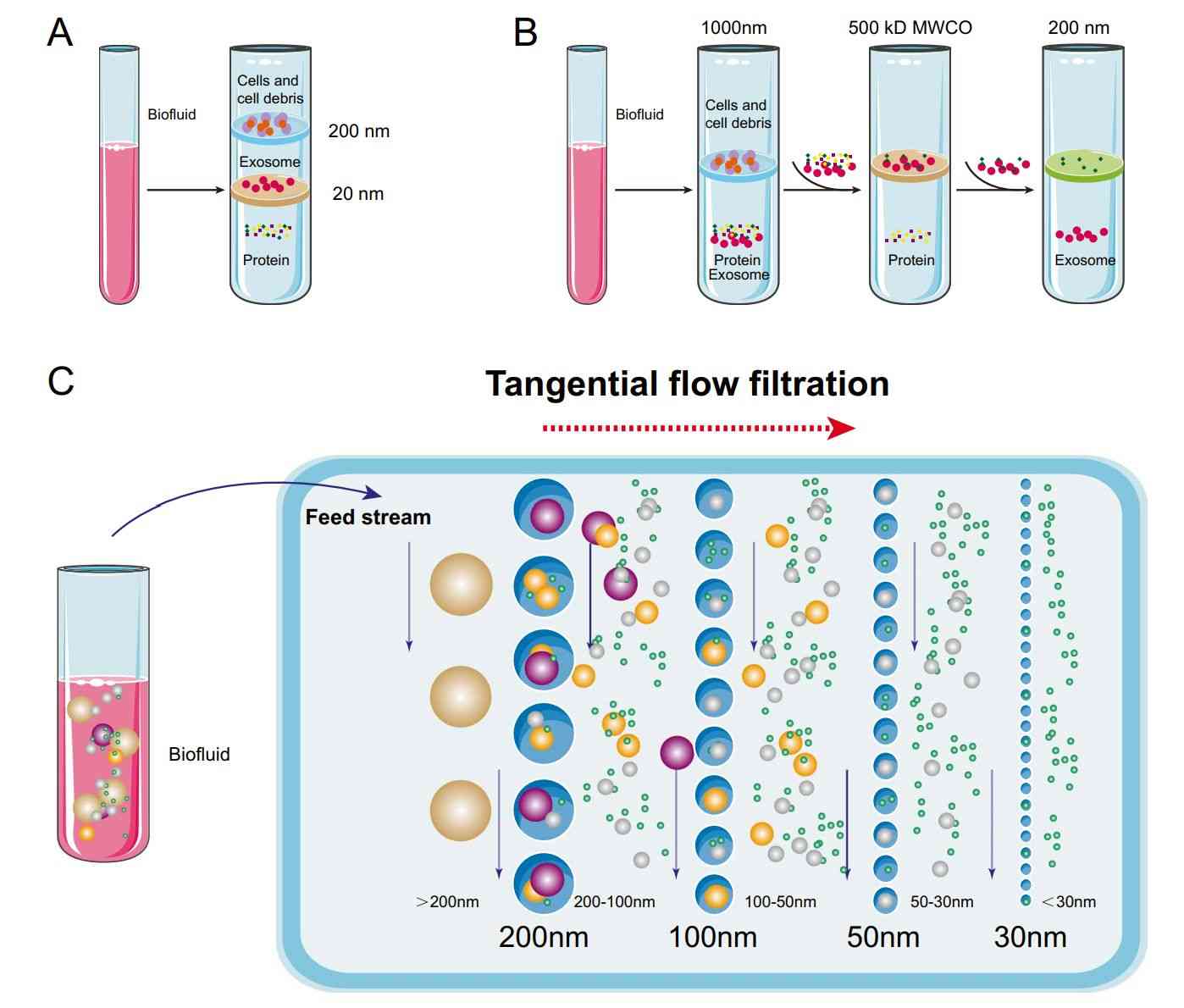
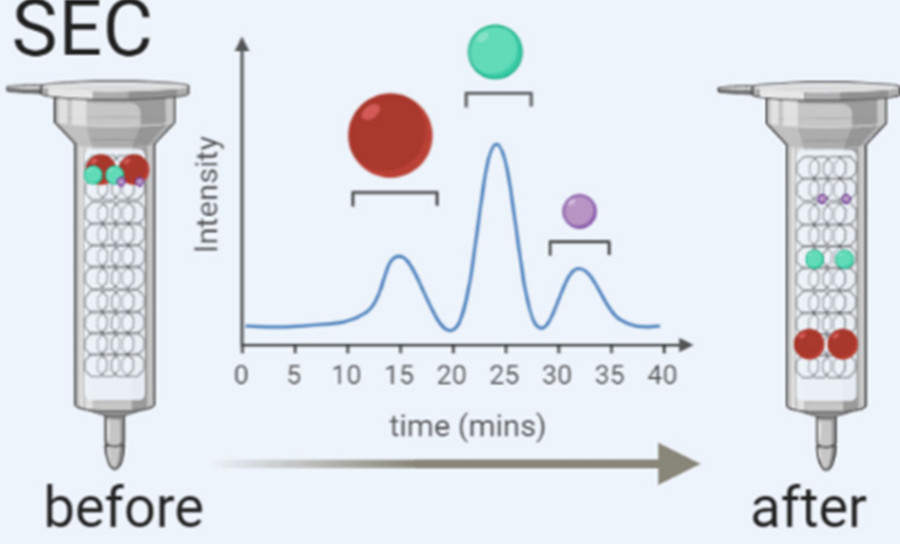 Figure 1. Principle of Size Exclusion Chromatography (SEC) for Extracellular Vesicle Separation. (Sidhom K, et al., 2020)
Figure 1. Principle of Size Exclusion Chromatography (SEC) for Extracellular Vesicle Separation. (Sidhom K, et al., 2020)
Our SEC-Based Exosome Purification Workflow
At Creative Biostructure, we have developed a robust and flexible SEC-based purification platform tailored to isolate high-purity, functionally intact exosomes from a wide variety of biological sources. Our workflow is designed to balance yield, purity, and reproducibility while preserving the native characteristics of extracellular vesicles.
Project Consultation & Method Selection
Each project starts with a consultation to align on goals and sample needs, followed by tailored EV purification using SEC, ultracentrifugation, ultrafiltration, or immunoaffinity.
Sample Submission & Acceptance Criteria
We accept diverse biofluids and culture supernatants, screening all samples for SEC compatibility. Submission guidelines help prevent degradation and maximize recovery.
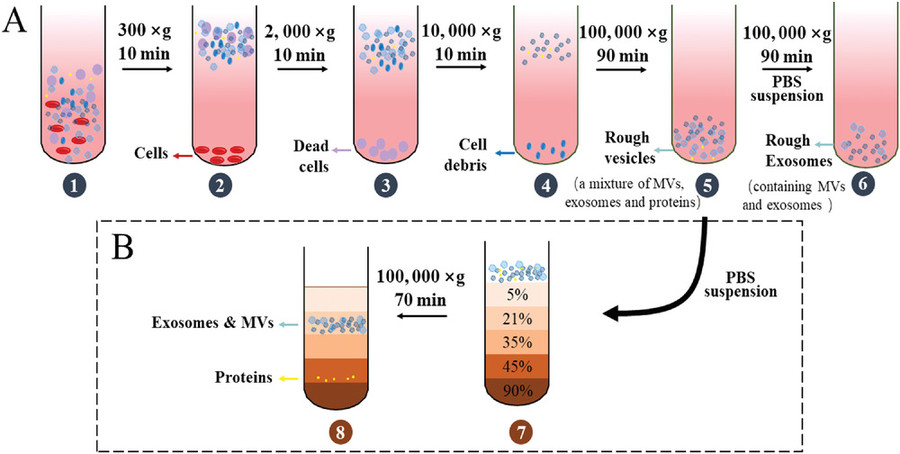
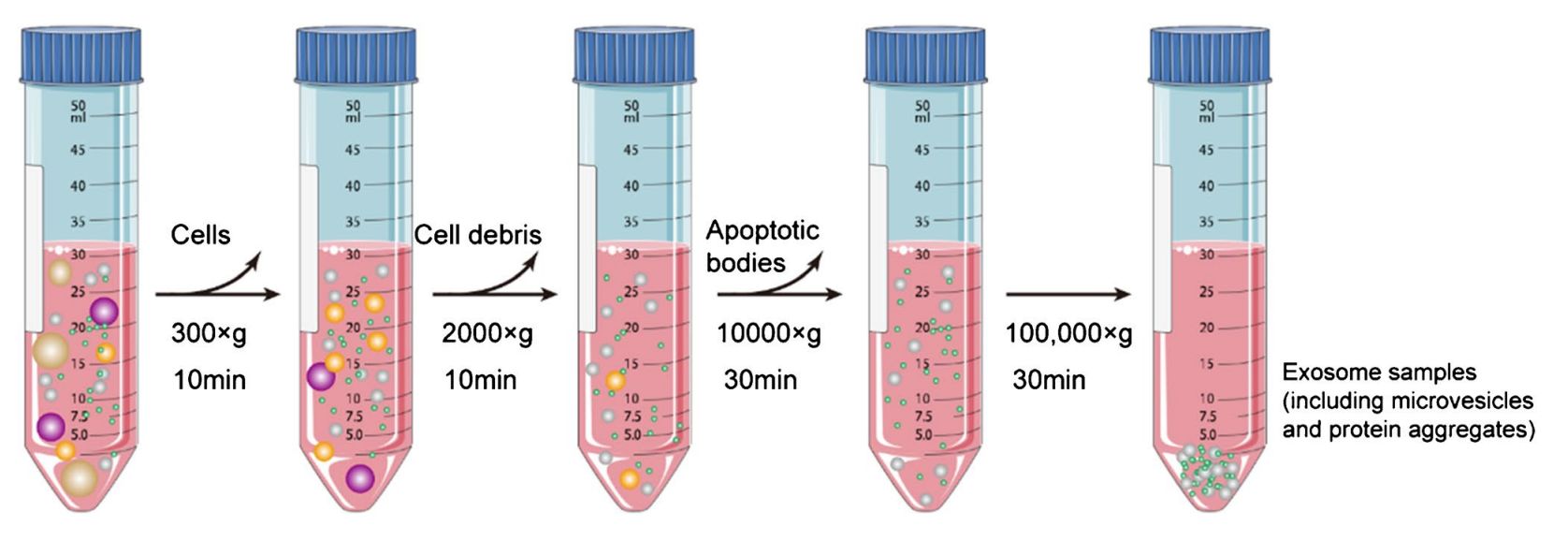
Pre-processing & Clarification
Before SEC, samples are cleared of debris, apoptotic bodies, and large particles by centrifugation and filtration, ensuring optimal column performance and gel protection.
SEC-Based Exosome Purification
Clarified samples are run on SEC columns with proprietary media: exosomes elute early, smaller molecules later. Standard or high-resolution columns and AFCs ensure purity and reproducibility.
Optional Enrichment & Purification Modules
For higher yield or purity, SEC can be combined with ultracentrifugation, ultrafiltration, or affinity capture, ideal for isolating exosomes from plasma, serum, or complex media.
Optional Quality Control & Characterization
We verify purification with NTA for size/concentration, TEM for morphology, BCA/Bradford for protein levels, and Western blot for markers and purity checks.
Final Deliverables
Clients receive purified exosomes in PBS or custom buffer, with optional QC report, full documentation, and storage/handling guidance for downstream use.
Our team ensures that each batch is handled with precision and consistency, offering the reliability required for biomarker studies, drug delivery research, or advanced omics profiling.
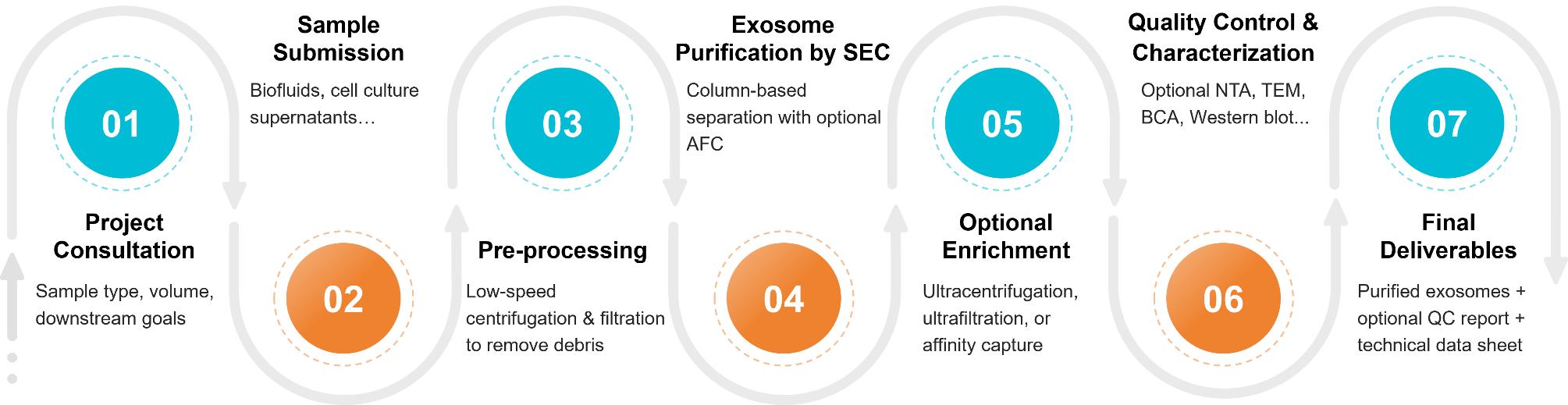 Figure 2. Project Workflow for Exosome Purification Using Size Exclusion Chromatography. (Creative Biostructure)
Figure 2. Project Workflow for Exosome Purification Using Size Exclusion Chromatography. (Creative Biostructure)
Sample Requirements
Our SEC-based exosome purification service is compatible with a wide spectrum of biological materials. For optimal recovery and purity, please follow the guidelines below when preparing your samples:
Accepted Sample Types
- Conditioned Cell Media: Derived from both adherent and suspension cell cultures.
- Mammalian Tissue-Derived Samples: Including brain, liver, tumor, and other soft tissues after enzymatic dissociation.
- Biofluids: Such as serum, plasma, saliva, urine, CSF, and ascites.
- Plant: Isolated from fruits, vegetables, herbs, flowers, seeds or plant suspension cultures.
- Fungal Vesicular Secretions: Obtained from yeast, molds, or filamentous fungal strains.
- Bacterial EV Samples: Secreted by Gram-positive and Gram-negative species under aerobic or anaerobic conditions.
- Parasitic EV Samples: Extracted from protozoan pathogens.
Recommended Volume
- Biofluids: ≥500 µL
- Cell media or digested tissue supernatants: ≥10 mL
Storage Conditions
All samples should be frozen at -80 °C and shipped on dry ice. Avoid repeated freeze-thaw cycles to preserve EV structure and function.
Case Study
Case: High-Efficiency Extracellular Vesicle Purification Using SE-FPLC
Background
To overcome the limitations of conventional exosome isolation techniques, researchers developed a fast and scalable method known as Size Exclusion Fast Protein Liquid Chromatography (SE-FPLC), which was evaluated alongside ultracentrifugation (dUC), density gradient separation (DG), and small-column SEC.
Key Observations
- Processing Time: SE-FPLC completed full isolation in ~18 minutes, significantly faster than dUC (≥8 hours).
- Yield and Purity:
- Achieved 88.5% EV recovery with over 99% protein contaminant removal.
- Clear separation of vesicular (CD81, CD9) and non-vesicular proteins (GAPDH, histone H3) confirmed by western blot.
- Sample Compatibility: Effective across Panc1, HEK293T, MSCs, and serum; serum EVs detectable from just 500 µL input.
- Structural Integrity: Cryo-EM imaging showed intact EVs (30-220 nm); NTA confirmed minimal particle presence in protein-rich fractions.
- Reproducibility: 16.4% across biological replicates, indicating robust performance.
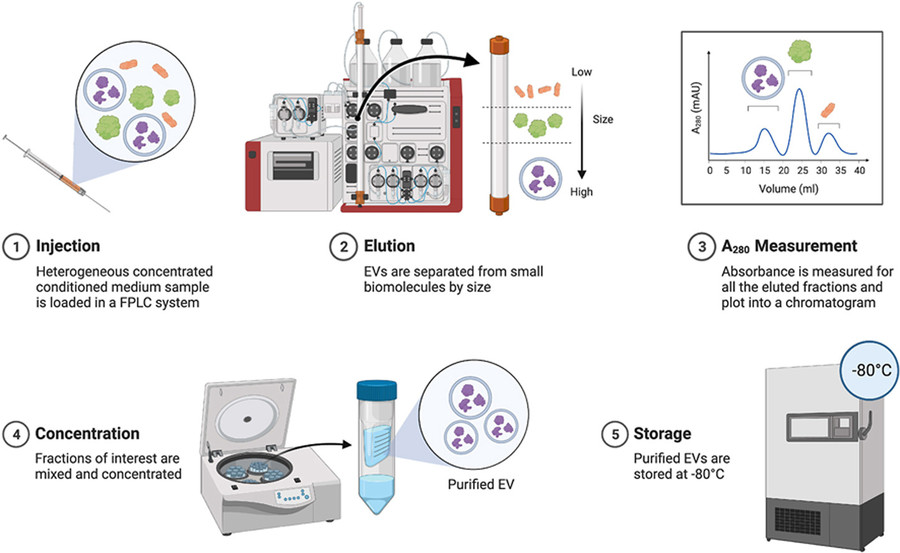 Figure 3. SE-FPLC enables efficient, high-yield EV isolation (<20 min, 88.47%) from various sources, including human and mouse serum and cell culture media. (Kapoor K S, et al., 2024)
Figure 3. SE-FPLC enables efficient, high-yield EV isolation (<20 min, 88.47%) from various sources, including human and mouse serum and cell culture media. (Kapoor K S, et al., 2024)
Conclusion
SE-FPLC provides an optimal combination of speed, purity, and scalability, making it well-suited for high-throughput exosome research and large-scale vesicle purification in laboratory settings.
At Creative Biostructure, we specialize in delivering high-quality, reproducible exosome preparations tailored to your research needs. Whether you're working with cell culture media, plasma, or complex biological fluids, our SEC-based purification platform ensures gentle and efficient isolation. Contact us to discuss your project and receive expert support from our experienced team.
References
- Sidhom K, Obi P O, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option?. International Journal of Molecular Sciences. 2020, 21(18): 6466.
- Jones M T, Manioci S W, Russell A E. Size exclusion chromatography for separating extracellular vesicles from conditioned cell culture media. Journal of Visualized Experiments (JoVE). 2022 (183): e63614.
- Kapoor K S, Harris K, Arian K A, et al. High throughput and rapid isolation of extracellular vesicles and exosomes with purity using size exclusion liquid chromatography. Bioactive Materials. 2024, 40: 683-695.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.