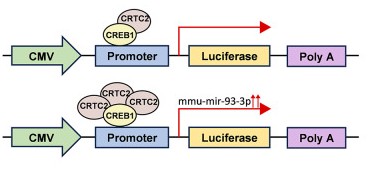
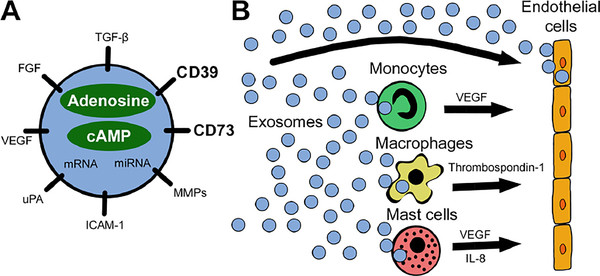
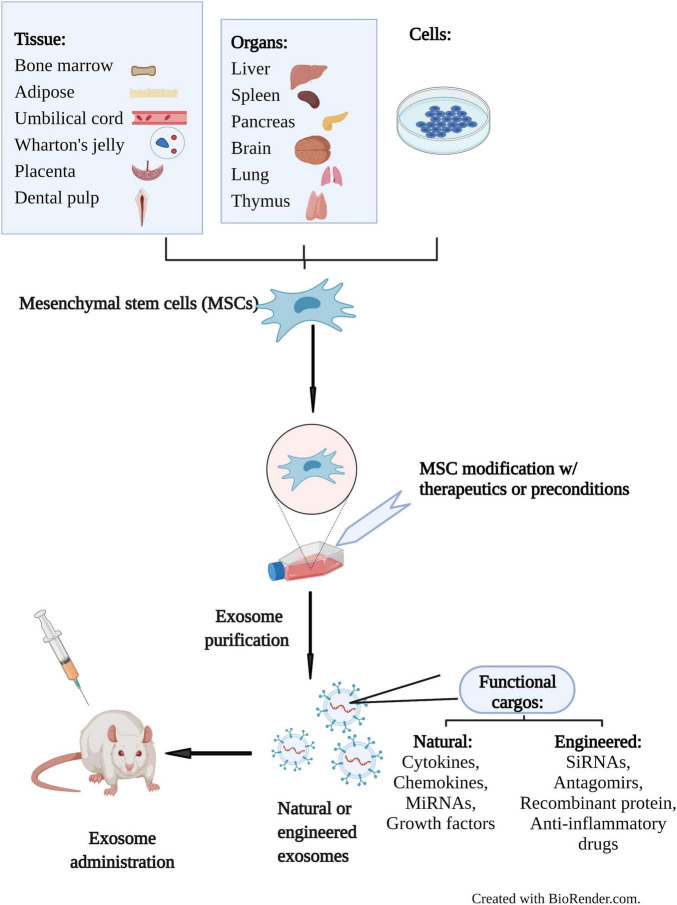
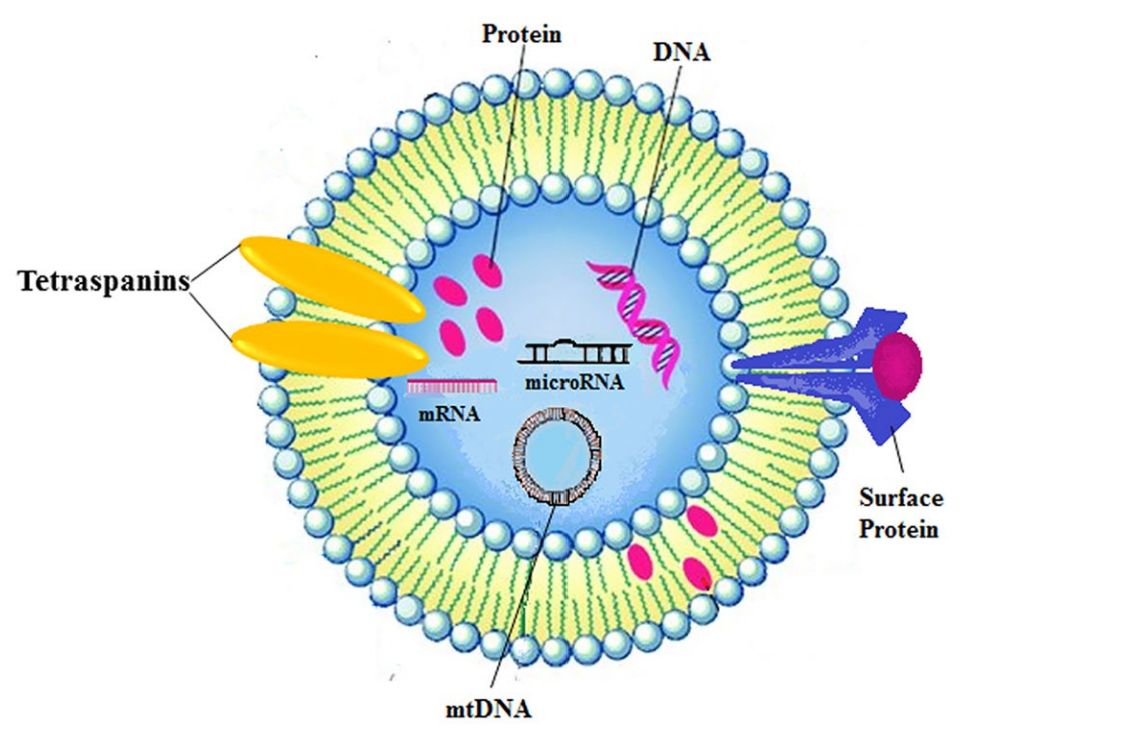
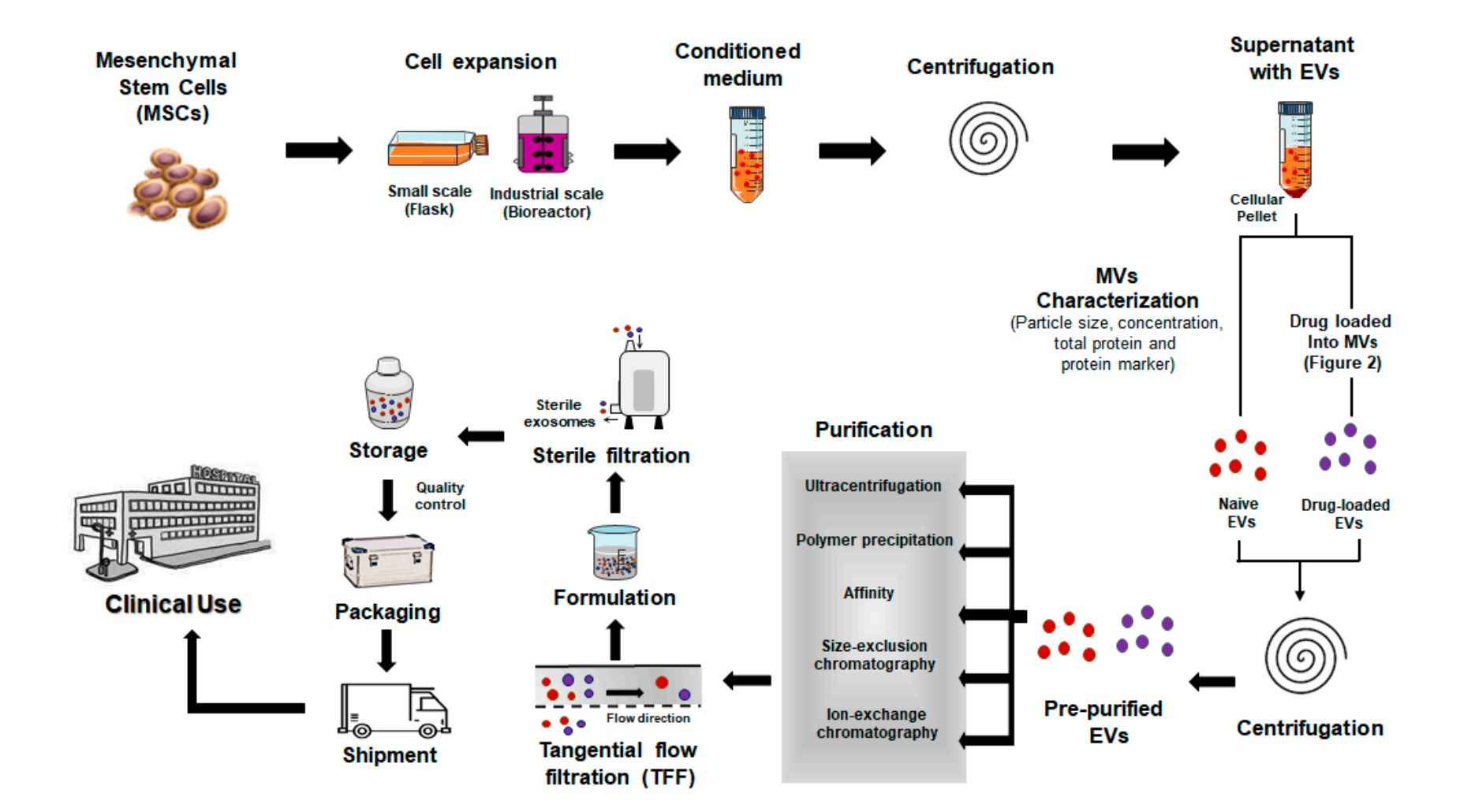
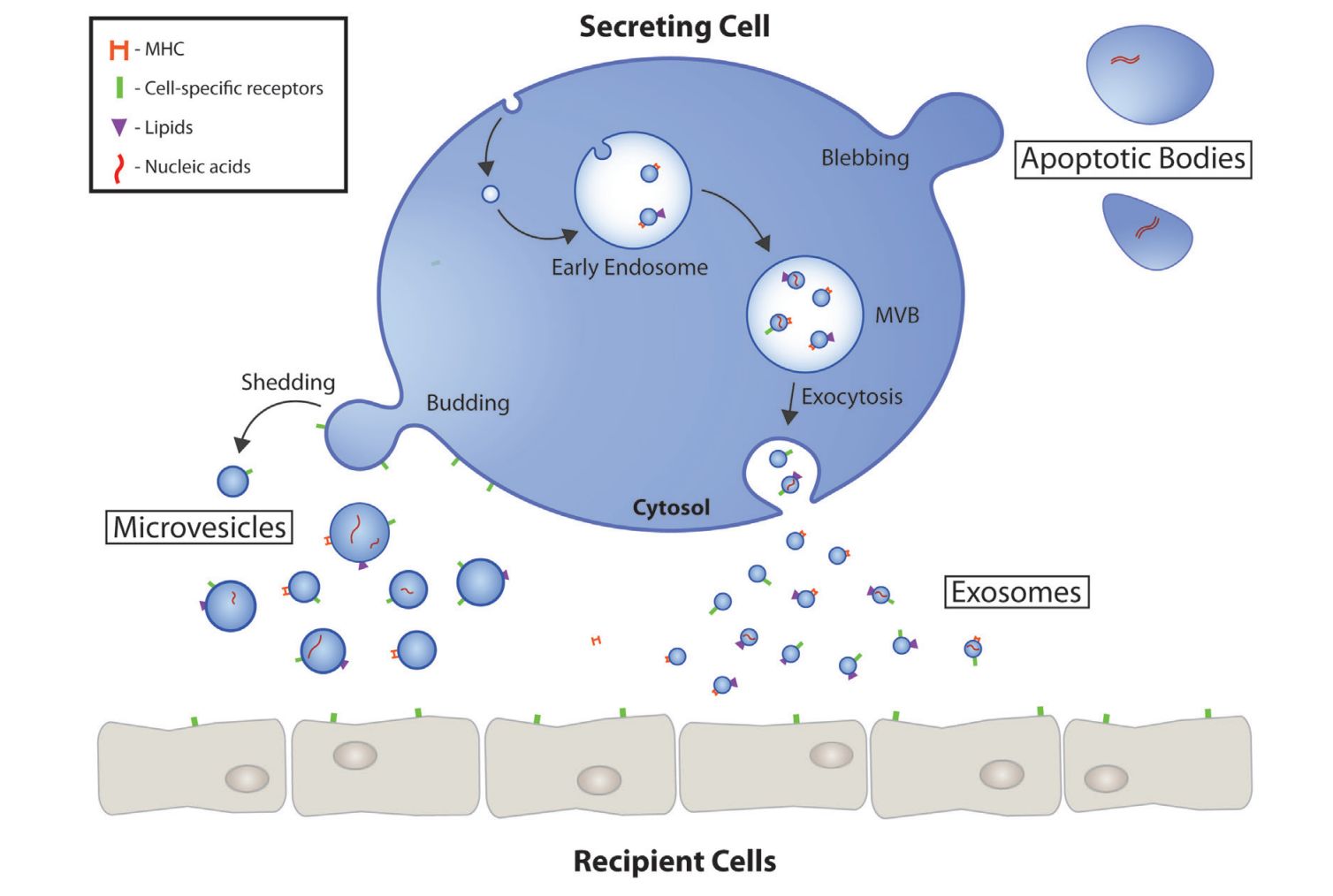
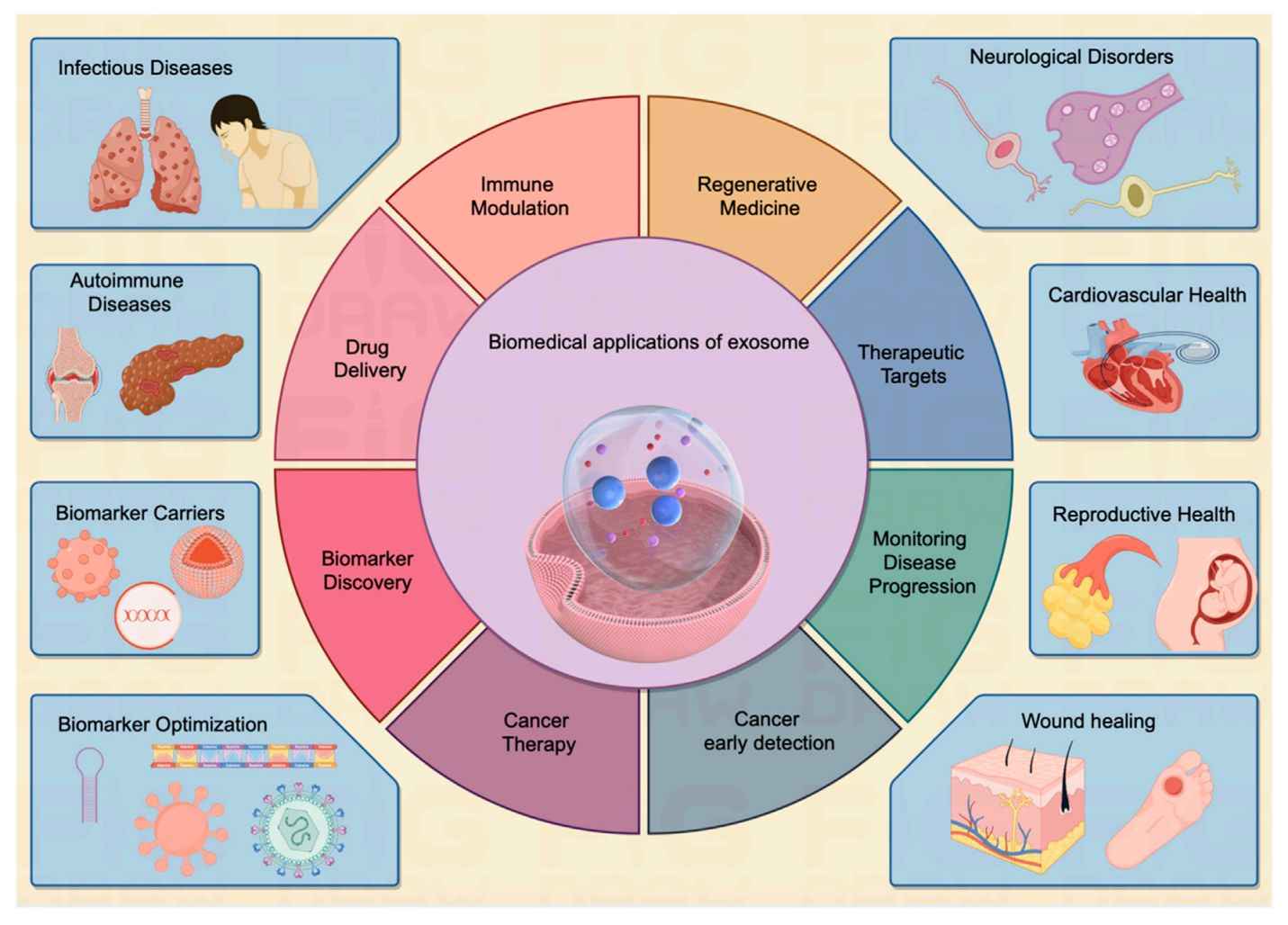
Exosome Cellular Functional Assays
Our Exosome Cellular Functional Assays are designed to answer one critical question: what do your exosomes actually do to target cells? This service platform moves beyond simple characterization to provide quantitative, hard data on the phenotypic changes exosomes induce.
We focus on the direct biological impact of your exosome in vitro treatment, providing precise measurements of everything from initial exosome cellular uptake and cellular internalization to downstream effects like proliferation, migration, and apoptosis.
Why Measuring Cellular Phenotype is the Critical Next Step?
Many studies stop at exosome isolation and cargo analysis (e.g., RNA-Seq). However, this only reveals the potential for a biological effect. Our cellular assays provide the essential proof of function.
- Validate Therapeutic Potency: Does your exosome-based therapeutic actually inhibit tumor growth (via apoptosis assays) or promote tissue repair (via migration assays)?
- Elucidate Disease Mechanisms: Does the exosomes cellular communication from diseased cells cause a malignant phenotype (e.g., invasion) in healthy cells?
- Confirm Cargo Activity: This is the definitive step to link a specific cargo (like a miRNA) to a physical, measurable change in cell behavior.
Quantifying the cellular phenotype validates your exosome's function and provides the high-impact data needed for publication or clinical advancement.
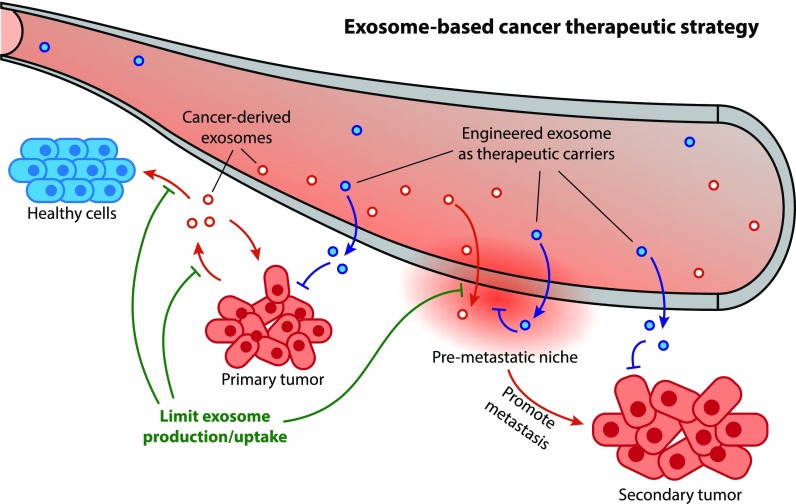
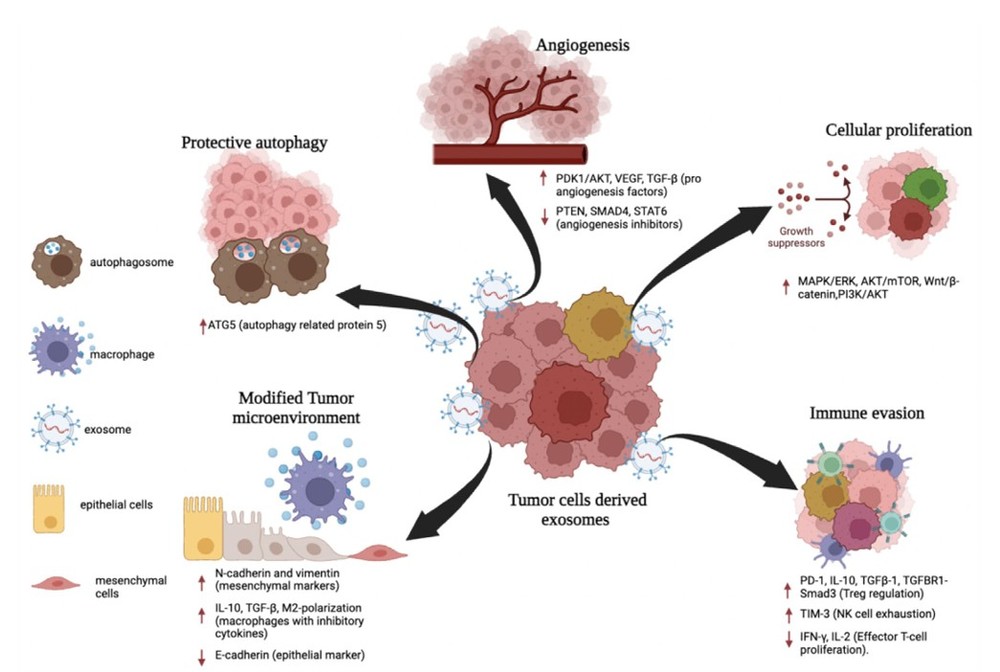 Figure 1. Exosomes promote cancer metastasis through proliferation, immune evasion, angiogenesis, autophagy, and microenvironment modification. (Aakel N, et al., 2025)
Figure 1. Exosomes promote cancer metastasis through proliferation, immune evasion, angiogenesis, autophagy, and microenvironment modification. (Aakel N, et al., 2025)
Platforms of Exosome Cellular Functional Assays
We offer a comprehensive portfolio of assays, each designed to answer a specific biological question. Our platforms utilize state-of-the-art technologies, from high-content imaging to real-time cell analysis.
Exosome Cellular Uptake and Internalization Assays
Before testing function, you must confirm your exosomes are being internalized. We track and quantify exosome cellular uptake.
- Technology Platforms:
- Fluorescent Labeling: We label exosomes with lipophilic dyes (e.g., PKH, DiO, DiR) for tracking.
- Qualitative Analysis (Microscopy): Confocal Microscopy to visualize exosome internalization and subcellular localization.
- Quantitative Analysis (Flow Cytometry): Measures the percentage of uptake-positive cells and the mean fluorescence intensity (MFI), providing a quantitative measure of uptake efficiency.
- Mechanism of Action: We can also design studies to determine how cellular internalization of exosomes occurs through specific pathways, such as endocytosis or phagocytosis.
Cell Proliferation and Viability Assays
These assays determine the effect of your exosomes on cell growth and health.
- Technology Platforms:
- Metabolic Assays: CCK-8 (WST-8) or MTT assays measure the metabolic activity of the cell population, which correlates with cell number.
- DNA Synthesis Assays: The EdU assay directly measures new DNA synthesis, providing a precise snapshot of cells actively dividing.
- Applications: Ideal for oncology (testing anti-tumor effects) and regenerative medicine (testing pro-growth effects).
Cell Migration and Invasion Assays
Essential for cancer metastasis and wound healing research, these assays measure cell motility.
- Technology Platforms:
- Scratch (Wound Healing) Assay: We create a "scratch" in a confluent cell monolayer. The rate of "gap closure" due to cell migration is imaged and quantified over time.
- Transwell Migration and Invasion Assays: We measure cell motility through a porous membrane (migration) or a membrane coated with an extracellular matrix (invasion).
Apoptosis and Cell Cycle Assays
These flow cytometry-based assays elucidate how your exosomes influence cell death pathways and cell division.
- Technology Platforms:
- Apoptosis Assay: We use dual Annexin V / Propidium Iodide (PI) staining to precisely quantify cells in early and late apoptosis.
- Cell Cycle Analysis: Cells are stained with a DNA-binding dye to quantify the percentage of cells in each phase (G0/G1, S, and G2/M), revealing if exosomes cause cell cycle arrest.
Our End-to-End Project Workflow
Our project workflow is specifically tailored for phenotypic analysis, ensuring robust and reproducible results from high-content imaging, flow cytometry, and real-time analysis platforms.
What You Can Expect from Our Process
Dedicated Project Management
You will be assigned a dedicated PhD-level project manager as your single point of contact, providing regular progress updates from start to finish.
Typical Turnaround Time
While project-dependent, standard cellular assays (e.g., proliferation, migration) are typically completed within 2-4 weeks from sample receipt.
Flexibility & Customization
Our workflow is not rigid. We can customize protocols, develop assays for your specific cell models (e.g., human iPSC exosome in vitro cell model), or adapt to unique research goals.
Data Security & Confidentiality
All project data and client information are handled under strict confidentiality. We provide secure data transfer and sign Non-Disclosure Agreements (NDAs) upon request.
The Pathway to Your Phenotypic Data:
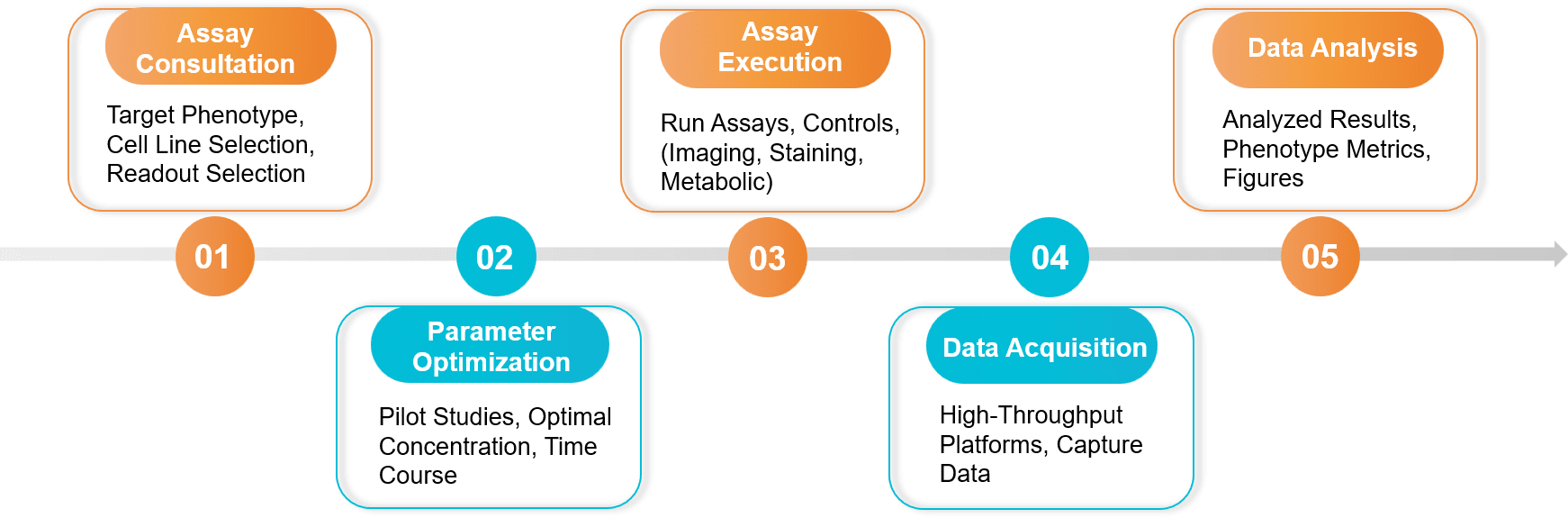 Figure 2. Exosome Cellular Functional Assay Project Workflow. (Creative Biostructure)
Figure 2. Exosome Cellular Functional Assay Project Workflow. (Creative Biostructure)
Sample Requirements
- Client-Provided Exosomes:
- Quantity: ≥ 1x10¹⁰ particles per assay (recommended, can be optimized).
- Purity: Purified exosomes are required. We strongly recommend providing characterization data (NTA, Western Blot).
- Buffer: Suspended in sterile PBS or a compatible cell culture-grade buffer.
- Recipient Cells:
- We can source most common cell lines (e.g., HeLa, MCF-7, HUVEC, RAW 264.7).
- We also have validated protocols for human iPSC exosome in vitro cell model studies, including iPSC-derived neurons, cardiomyocytes, and others.
Standard Deliverables
- A comprehensive project report detailing the experimental design, protocols, and parameters.
- All raw data (e.g., flow cytometry .fcs files, plate reader outputs, raw microscope images).
- All analyzed data, presented in publication-ready graphs and charts with statistical analysis.
- A final consultation with our scientific team to discuss the results and next steps.
Case Study
Case: Exosomes from Endothelial Progenitor Cells (EPCs) Protect Skin Cells from High-Glucose Induced Injury In Vitro
Background: Diabetic wound healing is slow, often because high glucose (HG) damages skin cells (keratinocytes). Researchers hypothesized that exosomes from endothelial progenitor cells (EPC-EXO) could protect these skin cells and promote healing.
Methodology: Therapeutic exosomes (EPC-EXO) were applied to skin cells (HaCaT) in a high-glucose (HG) environment. A panel of in vitro functional assays was then used to measure changes in cell proliferation, migration, and apoptosis to test the exosome's protective effects.
Key Findings:
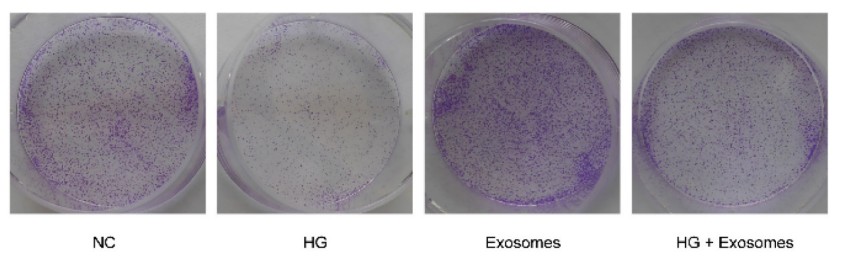 Figure 3. Exosomes derived from EPCs enhance the clone formation of HaCaT cells under HG condition. (Li P, et al., 2023)
Figure 3. Exosomes derived from EPCs enhance the clone formation of HaCaT cells under HG condition. (Li P, et al., 2023)
The in vitro functional assays provided a clear "before and after" picture. The high-glucose environment damaged the cells, but:
- Proliferation & Migration: Treatment with EPC-EXO significantly promoted the proliferation and migration of the high-glucose-treated skin cells.
- Inhibited Apoptosis: EPC-EXO treatment significantly inhibited the apoptosis that was being caused by the high-glucose stress.
Conclusion: This study is a perfect example of how exosome cellular functional assays are used to validate a therapeutic. The data from the proliferation, migration, and apoptosis assays all converged to prove that these exosomes were biologically active and functionally capable of rescuing cells from diabetic stress.
Ready to translate exosome findings into biology? We will recommend the right assays, tailor to your cell model, and deliver publication ready results. Contact us for a free consultation and a sample requirements checklist.
References
- Aakel N, Mohammed R, Fathima A, et al. Role of Exosome in Solid Cancer Progression and Its Potential Therapeutics in Cancer Treatment. Cancer Med. 2025 May;14(9):e70941.
- Li P, Hong G, Zhan W, et al. Endothelial progenitor cell derived exosomes mediated miR-182-5p delivery accelerate diabetic wound healing via down-regulating PPARG. Int J Med Sci. 2023 Feb 13;20(4):468-481.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.