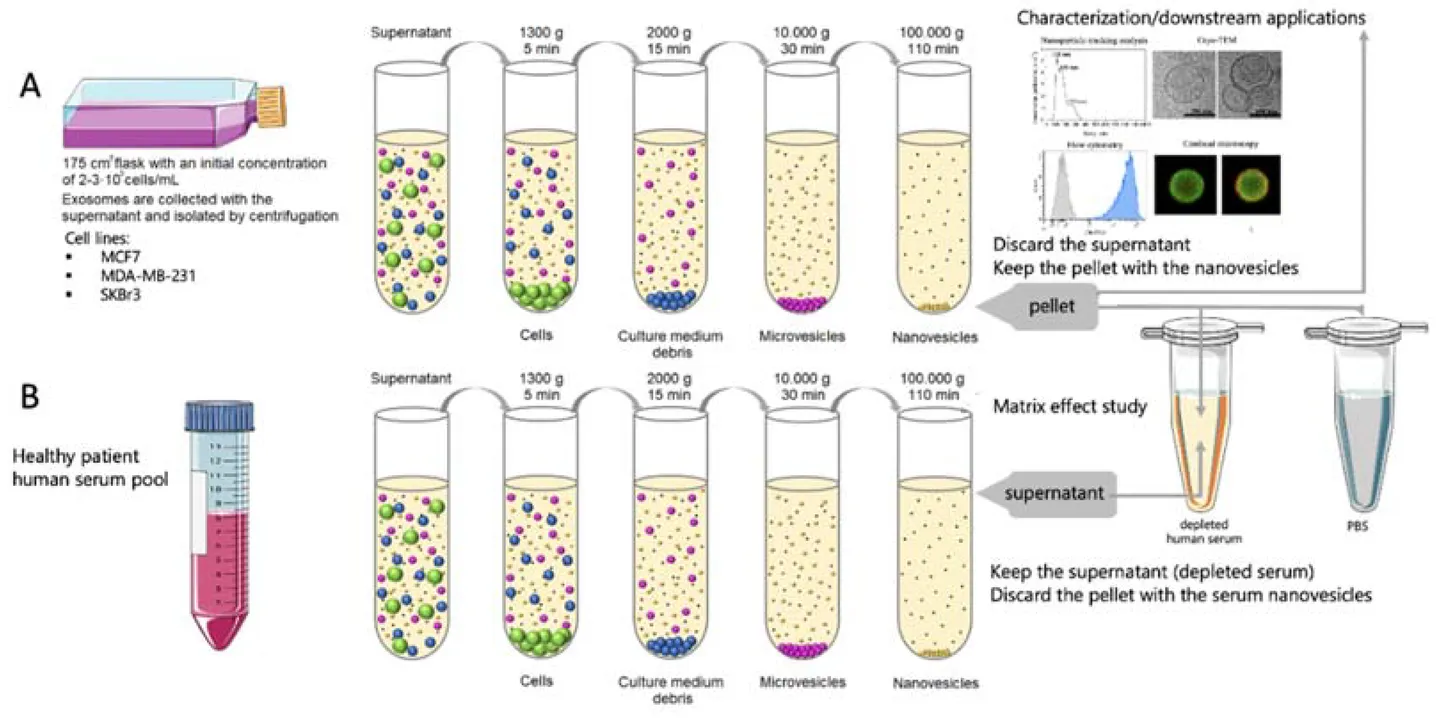
Bacterial Extracellular Vesicle (BEV) Isolation Service
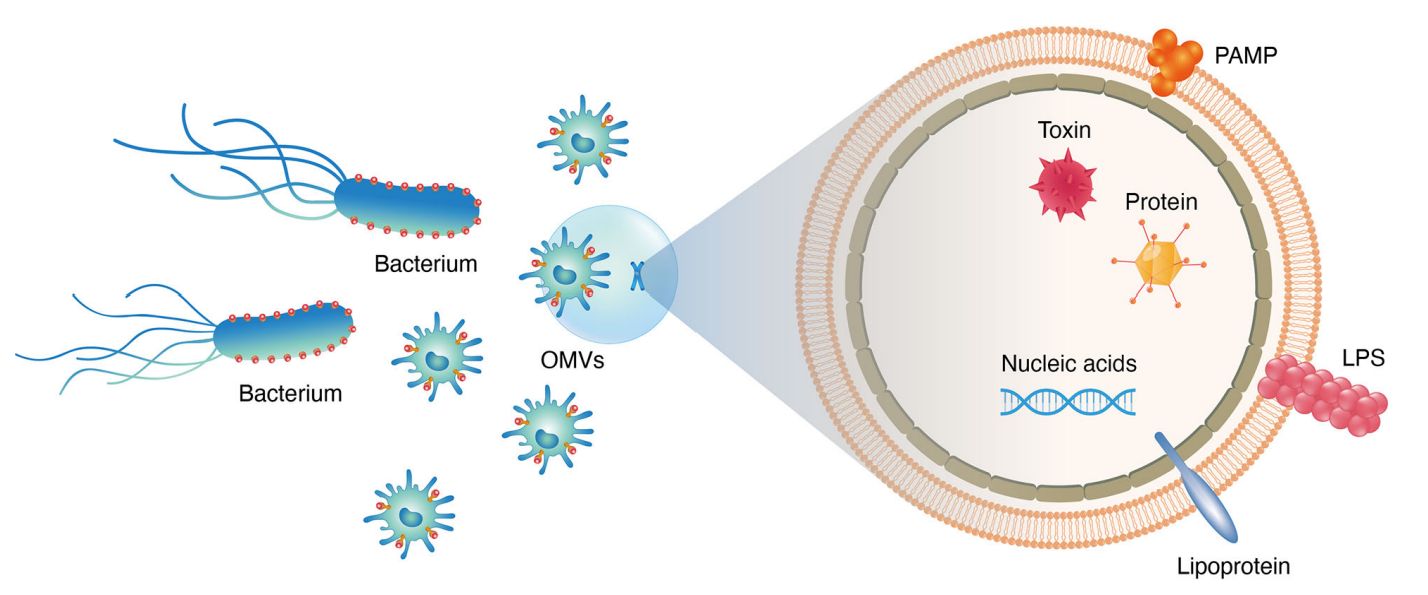
Bacterial extracellular vesicles (BEVs) are nano-sized membrane-bound particles naturally secreted by both Gram-negative and Gram-positive bacteria. These vesicles serve as critical mediators of intercellular communication, transporting proteins, lipids, nucleic acids, and metabolites across microbial communities and host organisms. As their biological significance becomes increasingly recognized in areas such as immunology, antibiotic resistance, and microbial pathogenesis, the demand for reliable BEV isolation has grown rapidly.
Creative Biostructure offers a professional BEV isolation service to support researchers in unlocking the full potential of bacterial vesicle-based studies. With extensive experience in extracellular vesicle workflows and microbial biology, we provide customizable solutions for BEV isolation, purification, and characterization.
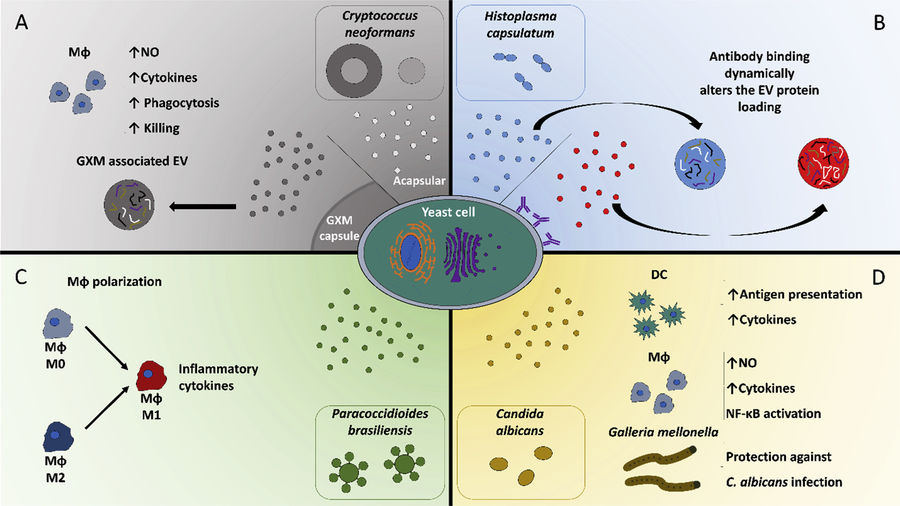
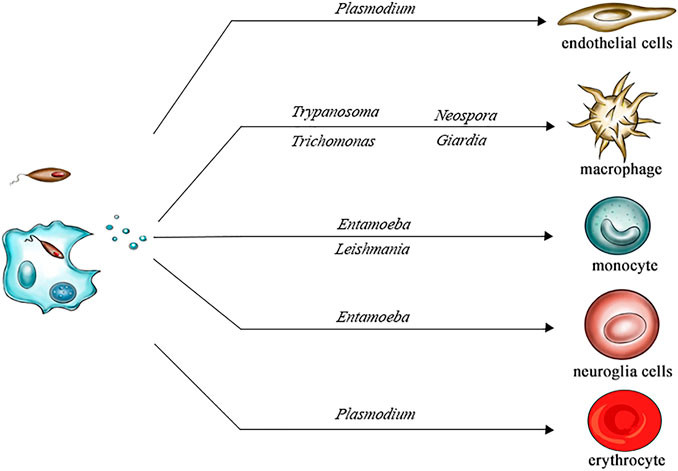
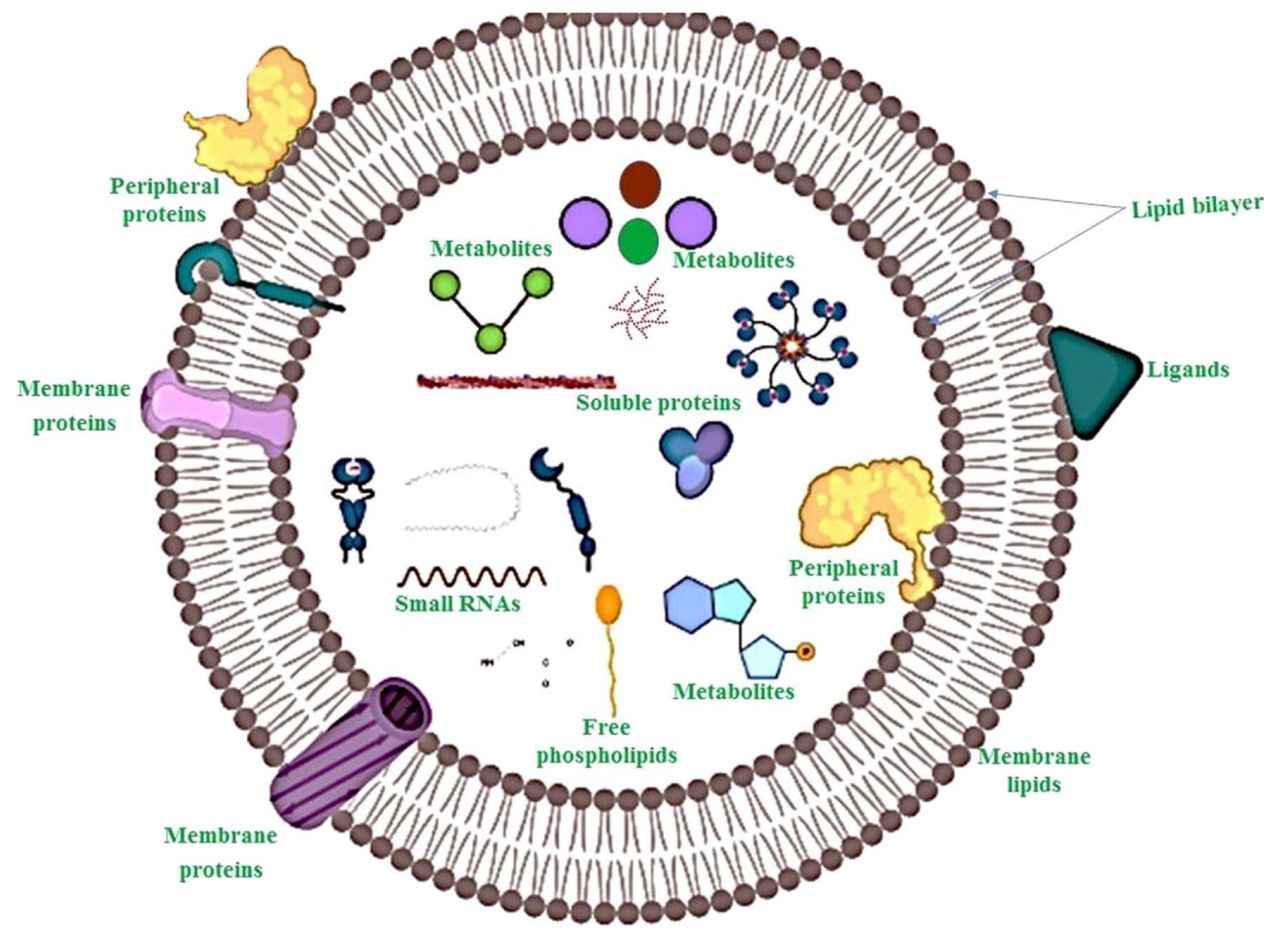
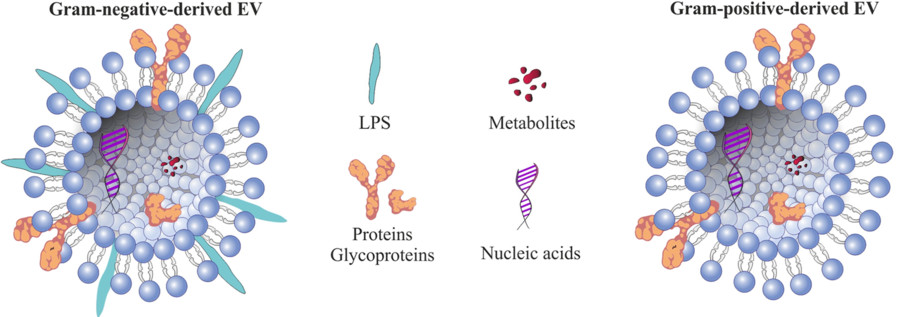 Figure 1. Structural Composition of Bacterial Extracellular Vesicles. (Ñahui Palomino R A, et al., 2021)
Figure 1. Structural Composition of Bacterial Extracellular Vesicles. (Ñahui Palomino R A, et al., 2021)
Why Study Bacterial Extracellular Vesicles?
Bacterial EVs play pivotal roles in:
- Microbe-host interactions: Modulating immune responses, triggering inflammation, and mimicking pathogenic molecules.
- Antibiotic resistance: Mediating horizontal gene transfer and harboring drug-degrading enzymes.
- Intra- and inter-species signaling: Delivering quorum-sensing molecules and toxins.
- Therapeutic applications: Acting as vaccine candidates and drug delivery vehicles.
- Microbiome research: Contributing to mucosal homeostasis and epithelial barrier regulation.
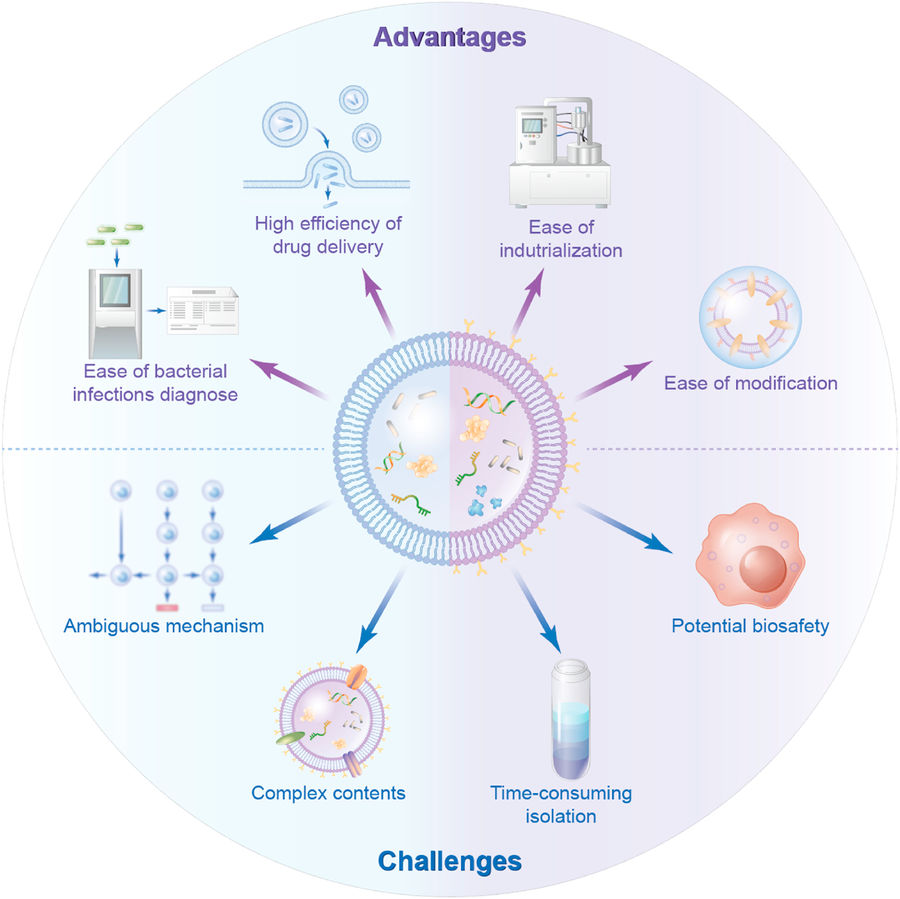 Figure 2. Advantages and Challenges of Bacterial Extracellular Vesicles. (Liu H, et al., 2022)
Figure 2. Advantages and Challenges of Bacterial Extracellular Vesicles. (Liu H, et al., 2022)
Our Bacterial EV Isolation Technologies
| Technology | Description | Applicable Scenarios |
|---|---|---|
| Ultracentrifugation (UC) | High-speed centrifugation method to pellet EVs based on density and size. Standard technique for initial vesicle recovery. | General-purpose BEV isolation from culture supernatants |
| Density Gradient Centrifugation | Separation of EV subpopulations using sucrose or iodixanol layers for improved purity and resolution. | Proteomics, biomarker discovery, downstream omics analysis. |
| Size Exclusion Chromatography (SEC) | Gentle column-based separation that preserves vesicle integrity while removing proteins and aggregates. | Functional studies, therapeutic development, in vivo applications |
| Tangential Flow Filtration (TFF) | Scalable membrane filtration system allowing efficient volume reduction and buffer exchange. | High-throughput processing, industrial-scale BEV production |
| Ultrafiltration (UF) | Size-based membrane filtration for rapid concentration and pre-cleaning. | Pre-processing step before SEC or gradient purification |
| Immunoaffinity Capture | Surface marker-based EV enrichment using specific antibodies or ligands. | Strain-specific BEV studies, host-pathogen interaction analysis |
| Enzyme-Assisted Extraction | Enzymatic softening of Gram-positive bacterial walls to facilitate vesicle release. | Isolation from Gram-positive strains (e.g., S. aureus, B. subtilis) |
| Hybrid Protocols (Custom) | Tailored combination of above methods to optimize yield, purity, and integrity for specific bacterial strains or applications. | Complex samples, microbiota-derived EVs, personalized project workflows |
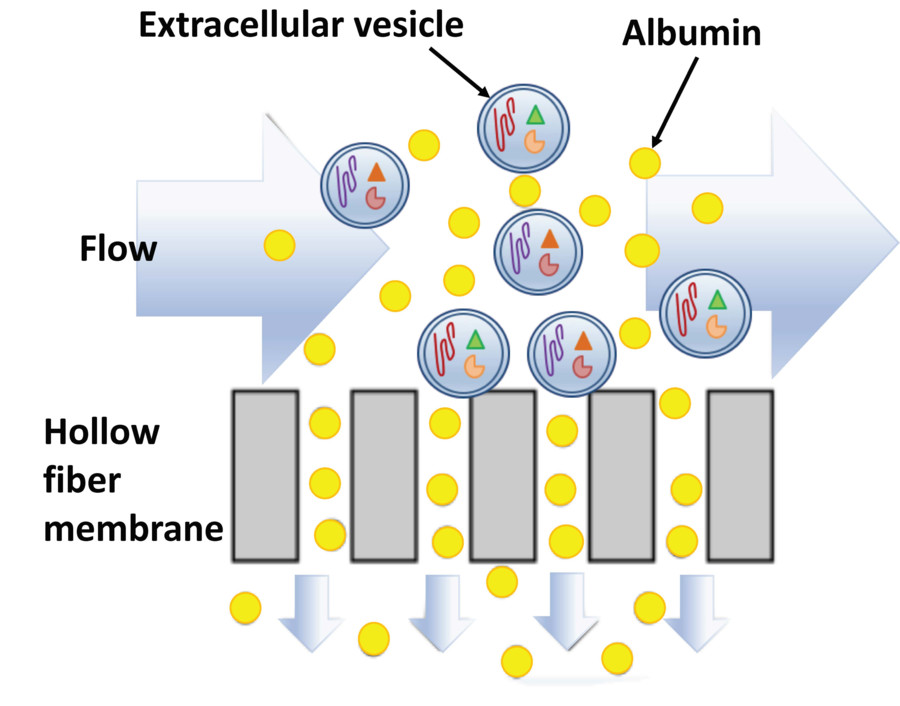
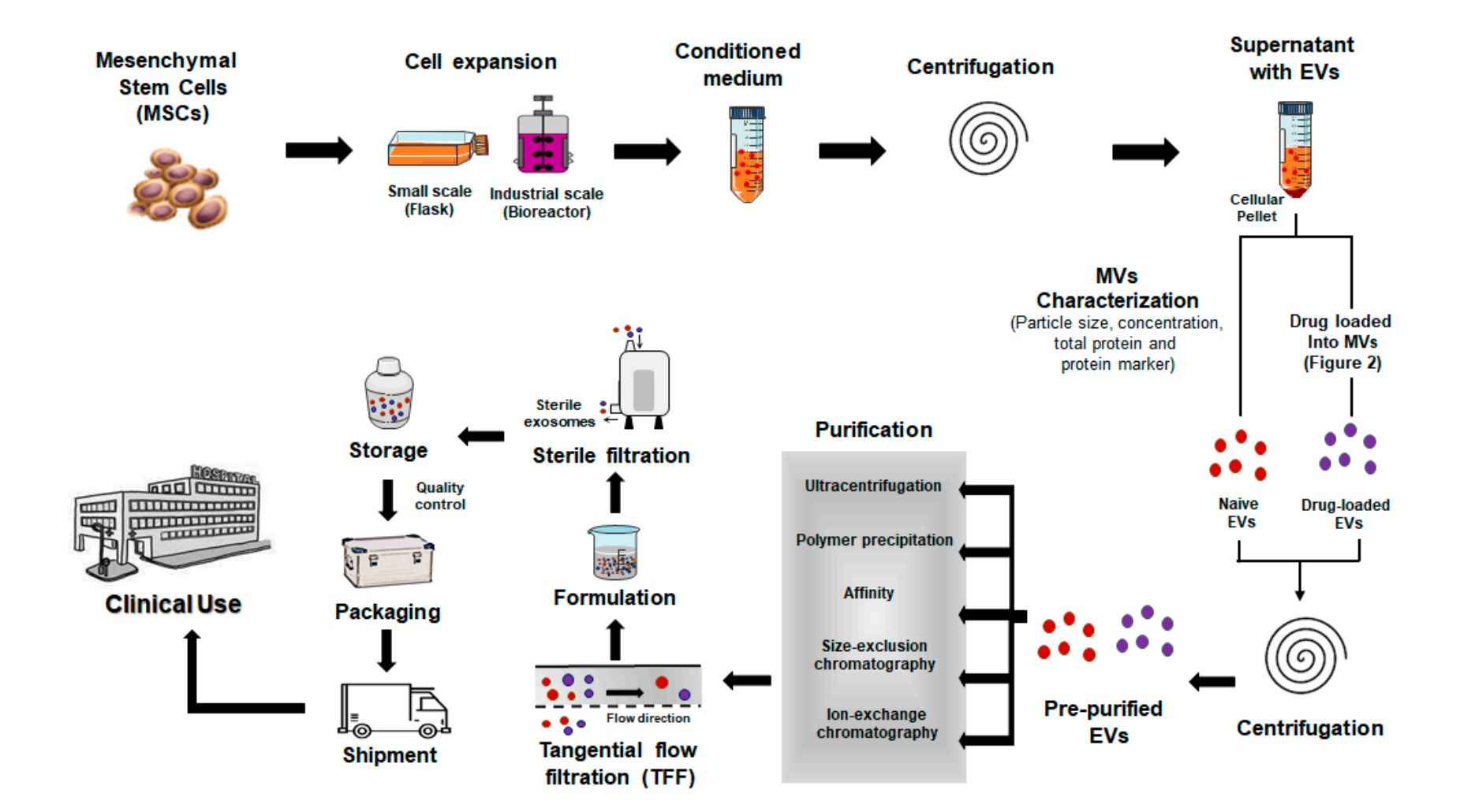
Our Bacterial EV Isolation Workflow
Creative Biostructure follows a well-defined, modular workflow to ensure efficient and reproducible isolation of high-quality bacterial EVs:
Initial Consultation
We begin by discussing your experimental goals, bacterial species, sample type, and intended downstream applications. Based on this information, we recommend the most appropriate isolation strategy.
Sample Submission
Clients can submit either live bacterial cultures, pre-collected culture supernatants, or other complex samples (e.g., biofluids containing bacteria). We provide detailed guidelines for sample handling and shipping.
Pre-processing
We remove intact bacteria, large particles, and cell debris through low-speed centrifugation and filtration. This ensures a clean starting matrix for vesicle isolation.
BEV Isolation & Enrichment
The core isolation step is carried out using one or more optimized techniques such as ultracentrifugation, gradient separation, or size exclusion chromatography. For Gram-positive bacteria, enzymatic pre-treatment is applied when needed to enhance vesicle release.
Purification & Buffer Exchange
BEVs are further purified and resuspended in a suitable buffer (e.g., PBS or a custom solution), ensuring stability and compatibility with downstream assays.
Quality Control & Optional Characterization
Each preparation undergoes rigorous quality assessment. Clients may opt for detailed analyses such as particle sizing (NTA), protein concentration, endotoxin levels, and TEM imaging.
Packaging & Delivery
Final deliverables include concentrated, ready-to-use BEVs along with a comprehensive technical report and QC documentation. All materials are shipped under temperature-controlled conditions to maintain vesicle integrity.
This workflow can be flexibly adapted for strain-specific optimization, scale-up production, or co-isolation of EVs from multiple bacterial species.
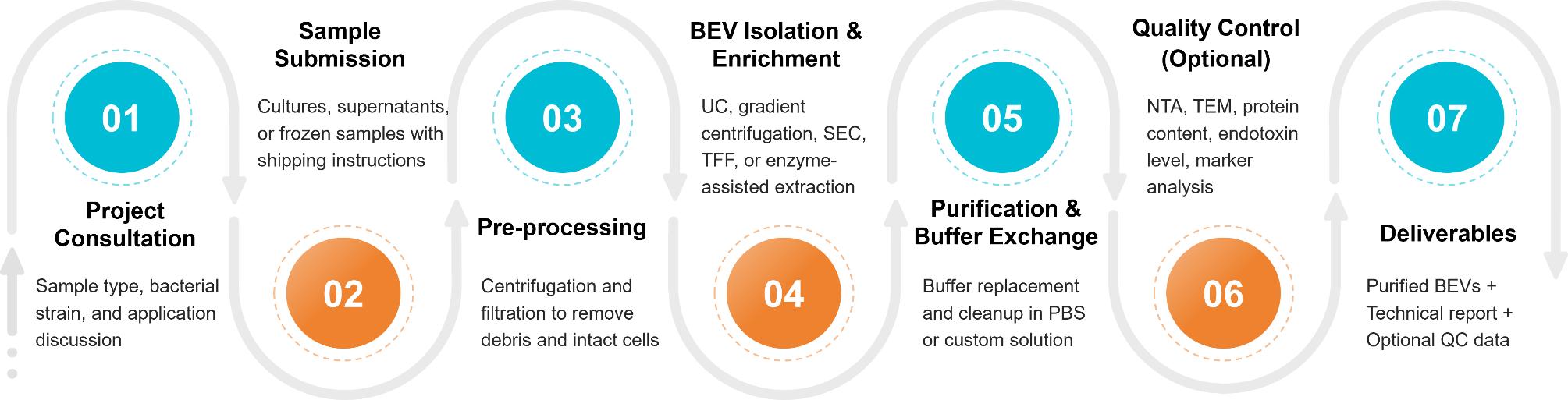 Figure 3. Bacterial Extracellular Vesicle Isolation Project Workflow. (Creative Biostructure)
Figure 3. Bacterial Extracellular Vesicle Isolation Project Workflow. (Creative Biostructure)
Sample Requirements
We offer bacterial extracellular vesicle (BEV) isolation from a wide range of Gram-negative and Gram-positive species under both aerobic and anaerobic conditions. Supported bacterial sources include:
- Gram-negative strains: Escherichia coli, Pseudomonas aeruginosa, Bacteroides spp…
- Gram-positive strains: Staphylococcus aureus, Bacillus subtilis, Lactobacillus spp…
We accept BEV samples from:
- Liquid bacterial cultures
- In vivo biological fluids (e.g., microbiota-associated samples)
Submission Guidelines:
- Minimum 50 mL of culture supernatant (contact us for lower-volume or alternative formats)
- Clearly label bacterial strain and growth medium used
- Ship purified samples on dry ice or fresh supernatants with cold packs
For special strain types or anaerobic culture requirements, please contact our team in advance.
Quality Control and Deliverables
To ensure high-quality vesicles and data reproducibility, we offer:
- Nanoparticle Tracking Analysis (NTA): Determines particle size distribution and concentration
- Transmission Electron Microscopy (TEM): Visualizes vesicle morphology and structural integrity
- Protein Quantification: Conducted via BCA or Bradford assay for total protein content
- Endotoxin Testing: Essential for immunology research and in vivo applications
- Marker Profiling: Western blot analysis of known bacterial EV markers (e.g., OmpA, LTA)
Deliverables:
- Purified bacterial extracellular vesicles in user-specified buffer (e.g., PBS)
- Technical report summarizing experimental procedures, protocols, and processing conditions
- Quality control report (if applicable) detailing NTA, TEM, protein content, and marker analysis
- Optional raw data files (NTA, TEM images, Western blot scans) for downstream interpretation
Customized QC packages and deliverable formats are available upon request.
Case Study
Case: Multistep Purification of Bacterial Extracellular Vesicles for Gut Microbiota Studies
Background
To support microbiota-related EV research, investigators established a standardized protocol for isolating BEVs from complex fermentation cultures. The approach aimed to ensure high purity and structural integrity for downstream immunological and morphological analyses.
Methods
- Bacterial cultures were centrifuged at 10,000 × g for 1 hour to remove intact cells.
- The supernatant was passed through a 0.22 µm sterile filter to eliminate remaining bacterial contaminants.
- BEVs were concentrated using 100 kDa regenerated cellulose ultrafiltration membranes to remove free proteins.
- Crude BEVs were pelleted by ultracentrifugation at 100,000 × g for 3 hours.
- Purification was performed using iodixanol-based density gradient centrifugation (10-60% w/v), followed by a second ultracentrifugation at 100,000 × g for 3 hours.
- BEVs were resuspended in PBS for characterization.
Characterization techniques included:
- TEM: Confirmed spherical morphology with ~200 nm diameter
- DLS & NTA: Validated size distribution and particle concentration
- NTA was preferred over DLS for improved accuracy in particles <150 nm
Conclusion
This study highlights a scalable and reproducible workflow for bacterial EV isolation using ultrafiltration and iodixanol gradient centrifugation. The combination of physical separation and advanced characterization methods enables reliable analysis of microbiota-derived vesicles for host-microbe interaction and immunomodulation studies.
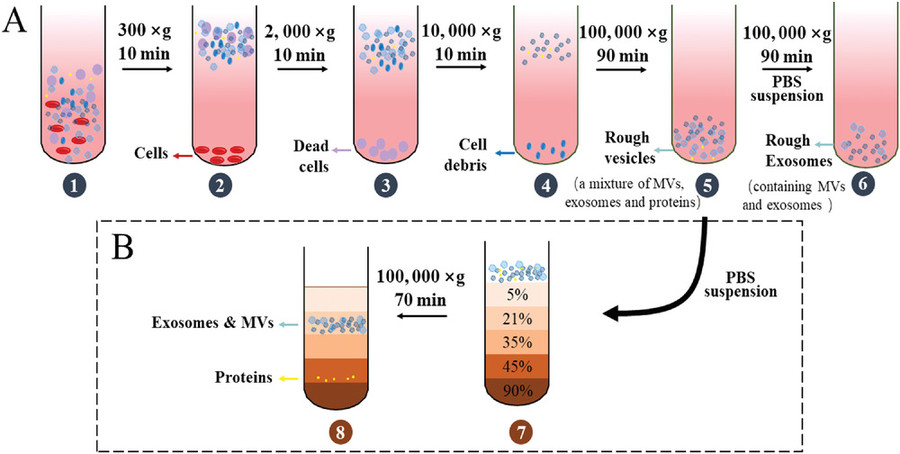
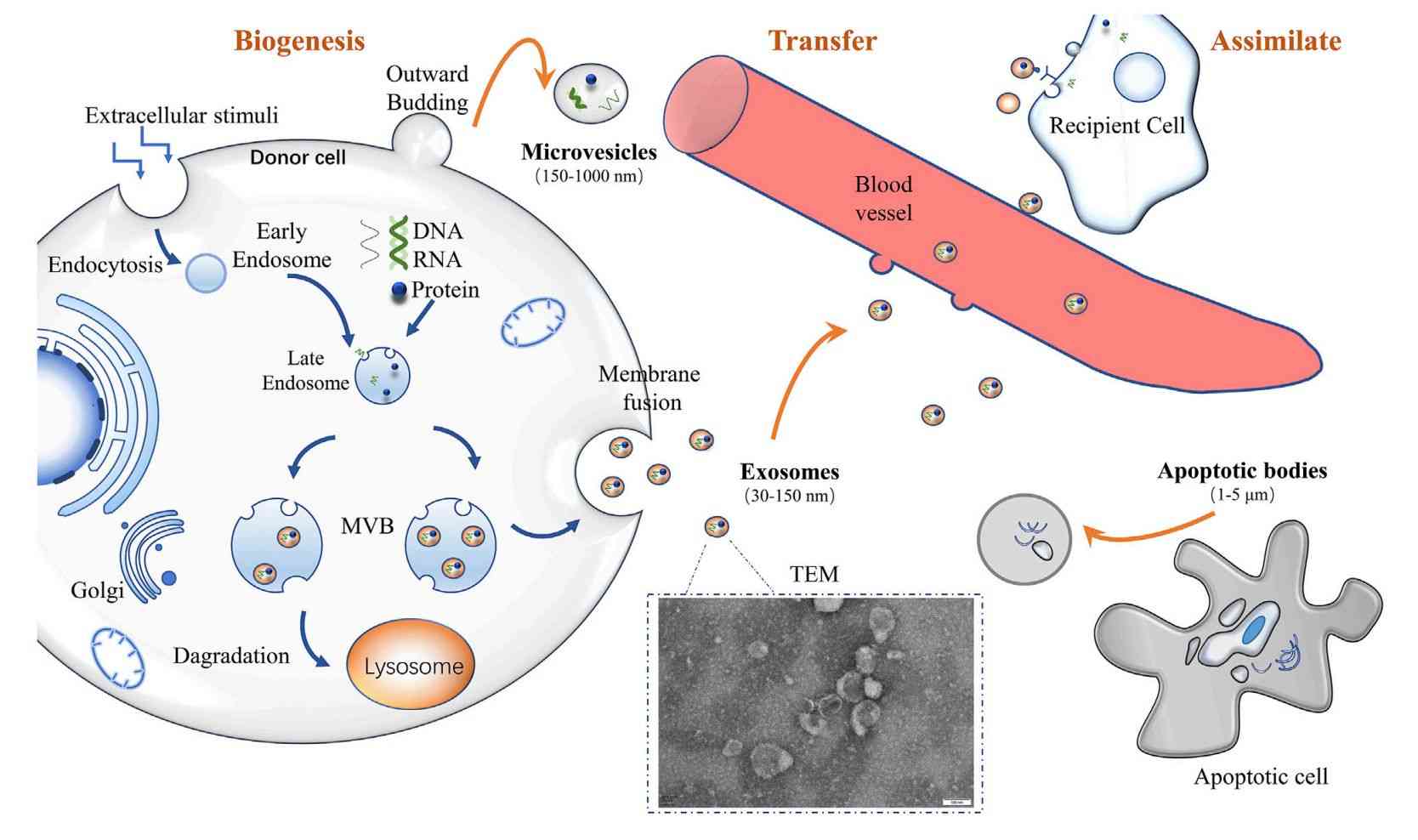
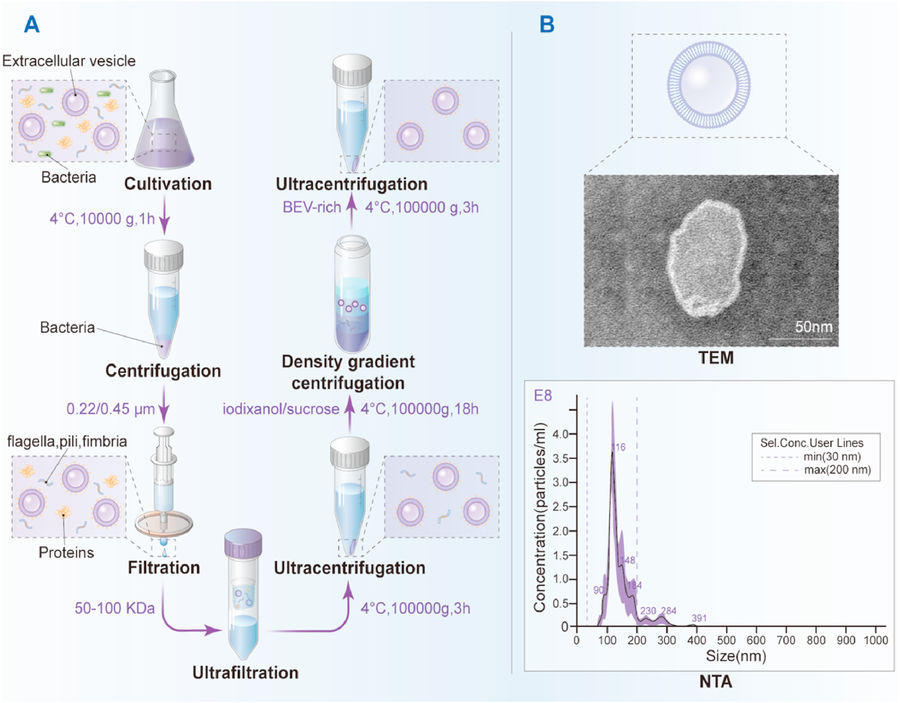 Figure 4. Workflow for Isolation and Characterization of Bacterial Extracellular Vesicles. (A) Schematic of BEV isolation using sequential centrifugation, filtration, ultrafiltration, and density gradient purification. (B) Representative characterization of Lactobacillus rhamnosus GG-derived vesicles by TEM and NTA. (Liu H, et al., 2022)
Figure 4. Workflow for Isolation and Characterization of Bacterial Extracellular Vesicles. (A) Schematic of BEV isolation using sequential centrifugation, filtration, ultrafiltration, and density gradient purification. (B) Representative characterization of Lactobacillus rhamnosus GG-derived vesicles by TEM and NTA. (Liu H, et al., 2022)
Ready to accelerate your bacterial EV research? Creative Biostructure offers flexible, strain-specific BEV isolation solutions backed by years of microbial and vesicle expertise. Contact us to discuss your project and request a free consultation.
References
- Ñahui Palomino R A, Vanpouille C, Costantini P E, et al. Microbiota-host communications: Bacterial extracellular vesicles as a common language. PLoS Pathogens. 2021, 17(5): e1009508.
- Xie J, Li Q, Haesebrouck F, et al. The tremendous biomedical potential of bacterial extracellular vesicles. Trends in Biotechnology. 2022, 40(10): 1173-1194.
- Liu H, Zhang Q, Wang S et al. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioactive Materials. 2022, 14: 169-181.
- Xie J, Haesebrouck F, Van Hoecke L, et al. Bacterial extracellular vesicles: an emerging avenue to tackle diseases. Trends in Microbiology. 2023, 31(12): 1206-1224.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.