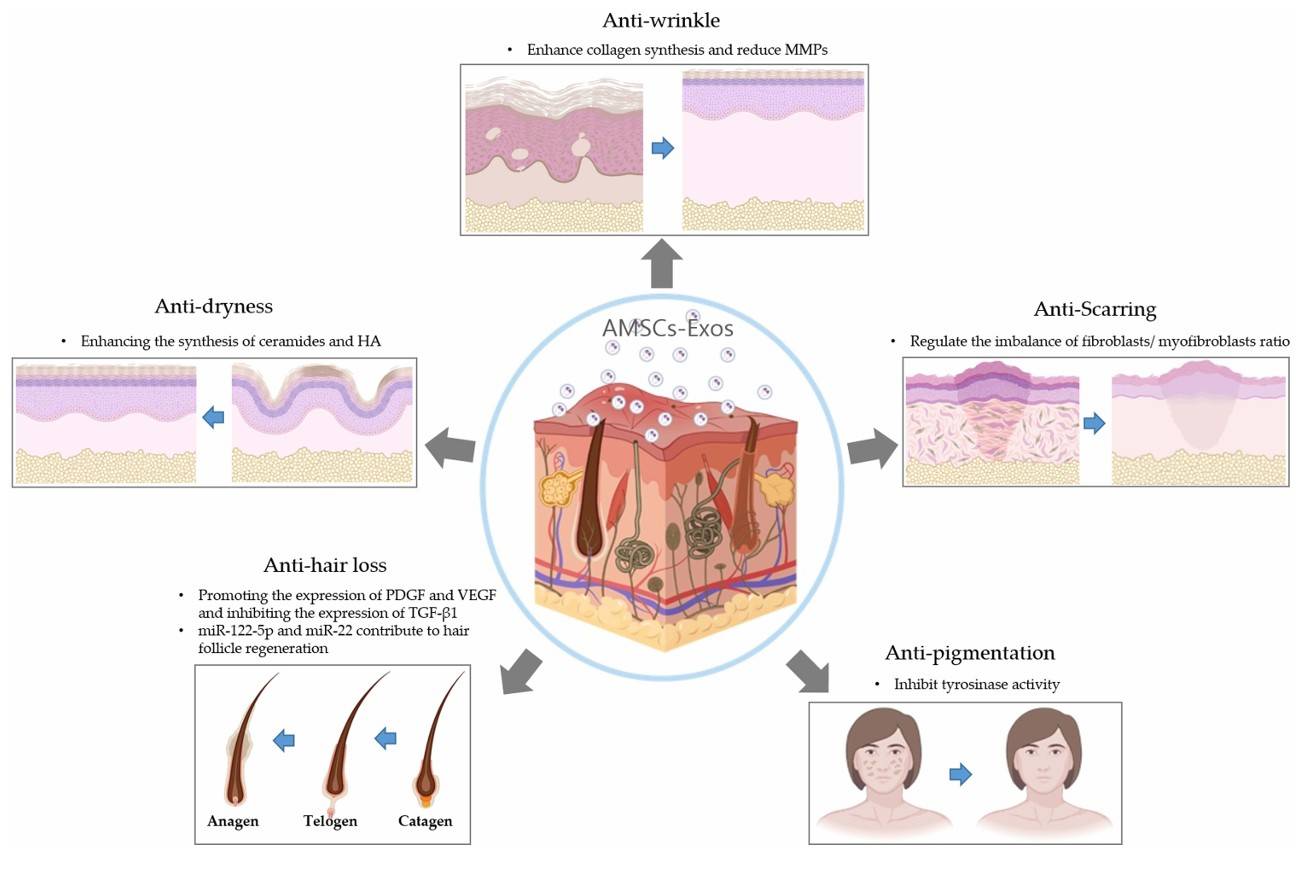
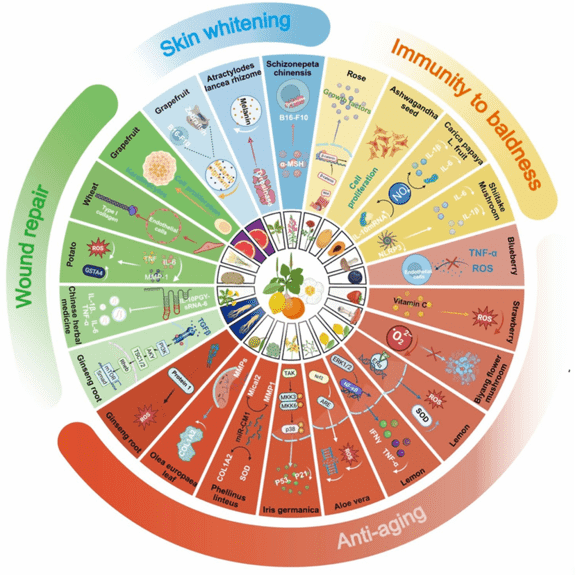
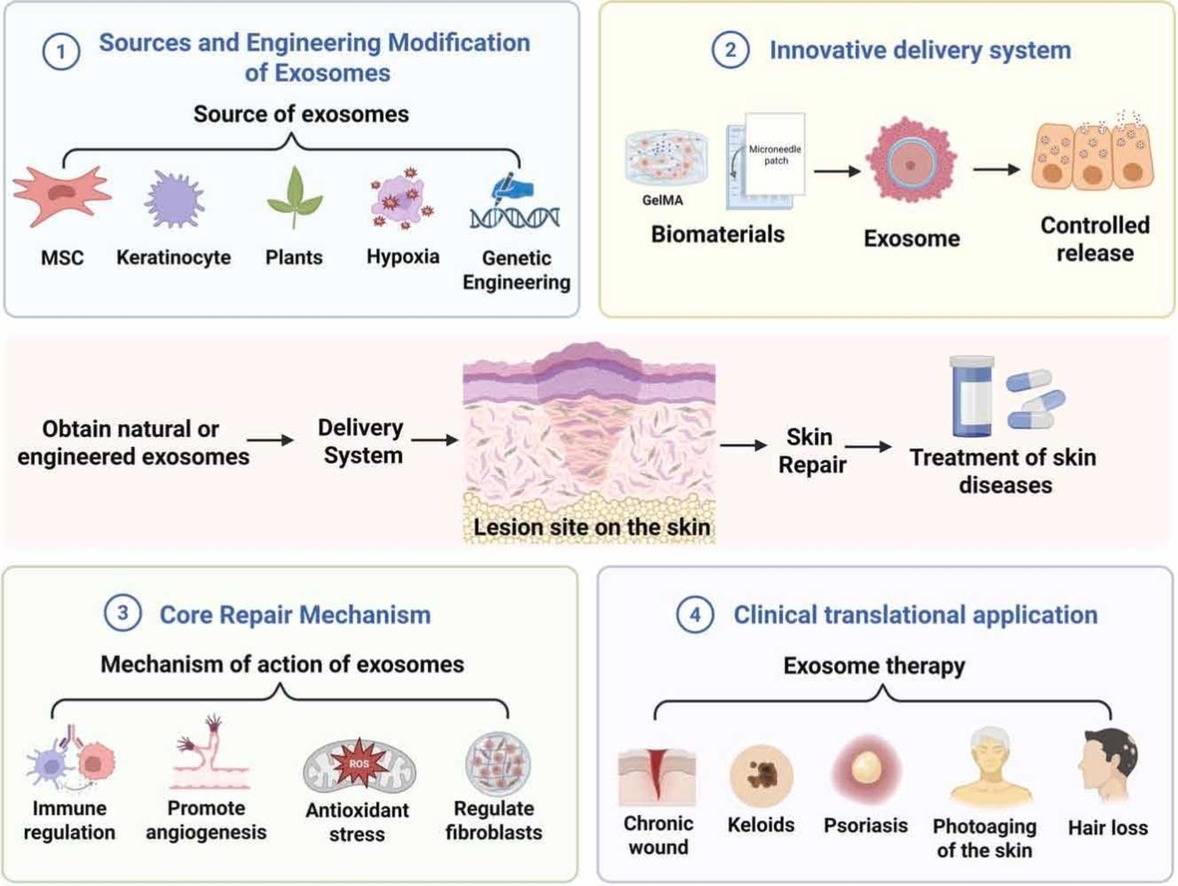
Cosmetic Exosome Formulation Development
The efficacy of an exosome skincare product depends entirely on its formulation. Raw exosomes are fragile; they degrade rapidly in water, aggregate at incorrect pH levels, and lose bioactivity when exposed to standard cosmetic preservatives. Turning a powerful biological ingredient into a shelf-stable consumer product requires precision engineering.
We provide specialized Cosmetic Exosome Formulation Development solutions. We solve the critical challenges of stability and delivery. From creating room-temperature stable lyophilized powders to engineering exosome-liposome hybrids for enhanced skin penetration, we help you build a finished product that retains the full regenerative power of the raw ingredient.
The Challenge of Keeping Vesicles "Alive" in a Bottle
Formulating with exosomes is unlike formulating with peptides or plant extracts. They are lipid-bound nanovesicles that require a biomimetic environment to survive.
- Shelf-Life Stability: In standard aqueous solutions, exosomes can degrade within days at room temperature. Developing a system that survives shipping and storage without cold chains is essential for commercial viability.
- Preservative Compatibility: Many common preservatives (like Phenoxyethanol) can disrupt the lipid membrane of exosomes, rendering them useless. Finding a "vesicle-friendly" preservative system is a key formulation hurdle.
- Skin Penetration: While exosomes are small, the skin barrier (stratum corneum) is formidable. Formulations must actively assist the exosomes in penetrating deep into the dermis without destroying their structure.
- Aggregation Prevention: Exosomes tend to clump together over time. Proper buffer optimization is needed to maintain them as monodisperse nanoparticles.
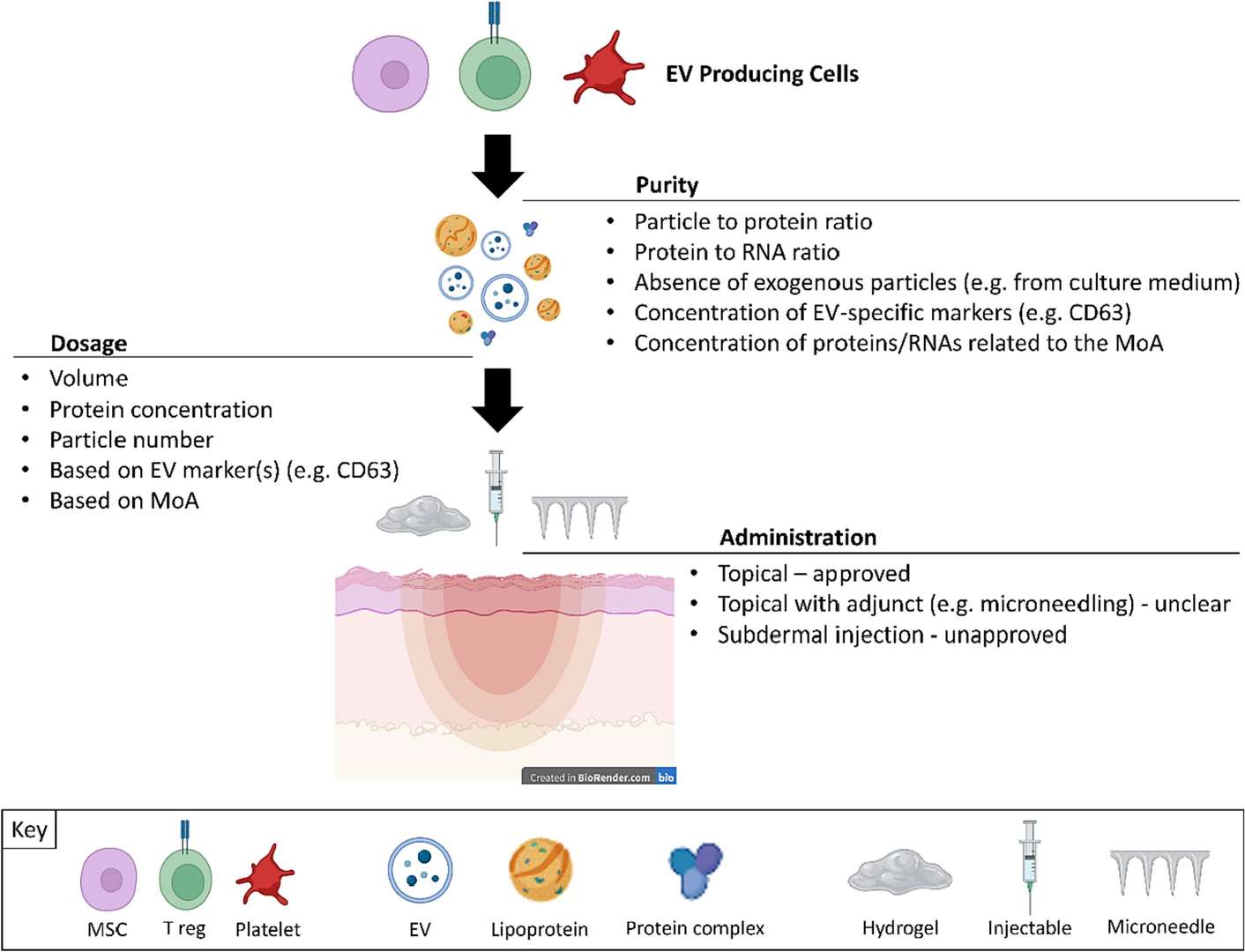 Figure 1. Key post-production parameters for formulating extracellular vesicle (EV) preparations in regenerative dermatology applications. (Davies OG, et al., 2023)
Figure 1. Key post-production parameters for formulating extracellular vesicle (EV) preparations in regenerative dermatology applications. (Davies OG, et al., 2023)
Our Formulation Development Workflow
We offer a systematic approach to stabilize your exosomes and optimize their delivery vehicle.
| Formulation Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Pre-Formulation Screening | Physical Stability Check: We screen your exosomes against cosmetic buffers and preservatives. We monitor Zeta Potential (surface charge) and Particle Size Distribution (PDI) to predict aggregation risks before formulation begins. | Exosome Particle Size and Concentration Analysis |
| Stabilization Strategy | Lyophilization Cycle Design: We develop custom freeze-drying protocols. We verify the morphology of the rehydrated exosomes using TEM/SEM to ensure the membrane remains intact after the freeze-thaw process. | Exosome Characterization by TEM |
| Delivery System Design | Advanced Vehicles: We engineer Exosome-Liposome Hybrids to improve skin lipid compatibility or encapsulate exosomes into Polymer Nanoparticles (for microneedles) to enhance penetration depth. | Exosome-Liposome Hybrid Vesicle Construction, Exosome-Polymer Hybrid Nanoparticle Engineering |
| Shelf-Life Validation | Real-Time Stability: We conduct accelerated aging tests. Crucially, we verify Batch-to-Batch Consistency and re-test Bioactivity (e.g., uptake efficiency) after storage to prove the product stays active on the shelf. | Exosome Quantification, Drug Delivery and Bioactivity Validation |
Core Technologies for Stable Skincare
We utilize advanced pharmaceutical technologies adapted for the cosmetic industry.
Optimized Lyophilization Protocols
The Gold Standard for Stability: To avoid cold chain logistics, we specialize in freeze-drying exosomes. We optimize the ratio of cryoprotectants to prevent "ice crystal damage" during freezing. The result is a porous cake that reconstitutes instantly into a clear, active serum upon adding water, ideal for high-end "fresh mix" product concepts.
Exosome-Liposome Hybrid Engineering
Boosting Penetration: We fuse exosomes with synthetic liposomes to create hybrid vesicles. This technique improves the colloidal stability of the exosomes and allows for the modification of the lipid surface to enhance interaction with skin lipids, significantly improving transdermal delivery efficiency compared to naked exosomes.
Hydrogel & Microneedle Integration
Sustained Release: For masks and patches, we formulate exosomes into temperature-sensitive hydrogels or dissolving microneedle arrays. These systems provide a sustained release of bioactive factors into the skin over hours, maximizing the therapeutic window for wound healing or anti-aging applications.
Application Spotlight: Hydrogel-Loaded Exosomes for Sustained Release
This analysis highlights how formulating exosomes into a functional hydrogel can solve the problems of rapid clearance and instability, creating a potent topical product.
Featured Technologies:
- Exosome Hydrogel Formulation
- In Vivo Efficacy Testing
Literature Interpretation:
Topical application of exosomes on chronic wounds is inefficient because liquid exosomes run off easily and degrade quickly. Researchers developed a bioactive, self-healing hydrogel (FHE hydrogel) and loaded it with exosomes derived from adipose mesenchymal stem cells. The hydrogel provided a sustained release of exosomes over time, protecting them from degradation. In a diabetic wound model, the exosome-loaded hydrogel significantly accelerated wound closure, promoted collagen deposition, and even induced hair follicle regeneration (a sign of complete skin restoration). This confirms that integrating exosomes into advanced hydrogel matrices is a superior formulation strategy for maximizing therapeutic efficacy and product stability.
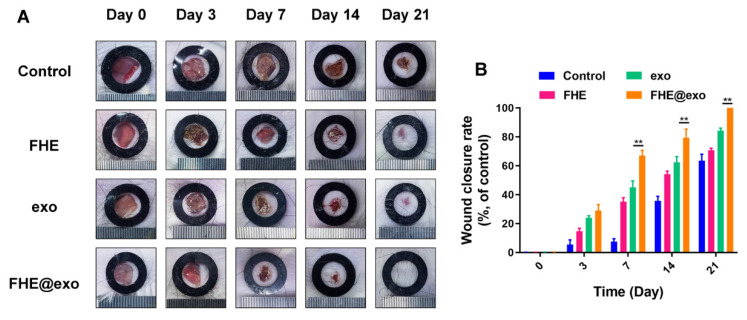 Figure 2. FHE@Exo hydrogel promotes wound healing. (A) Images of wound healing with treatments of FHE, exosomes, FHE@Exo, and control; (B) Comparison of wound closure rates among the groups. (Wang C, et al., 2019)
Figure 2. FHE@Exo hydrogel promotes wound healing. (A) Images of wound healing with treatments of FHE, exosomes, FHE@Exo, and control; (B) Comparison of wound closure rates among the groups. (Wang C, et al., 2019)
Start Your Formulation Project
Turn your raw ingredient into a shelf-stable, market-ready product.
How It Works: Our Project Pathway
 Figure 3. Our systematic approach to stabilizing exosomes and engineering effective delivery vehicles for commercial skincare products. (Creative Biostructure)
Figure 3. Our systematic approach to stabilizing exosomes and engineering effective delivery vehicles for commercial skincare products. (Creative Biostructure)
Ready to solve the stability puzzle for your exosome skincare line? Our formulation scientists are available for a free consultation to discuss your product requirements. Contact us today to discuss your project.
References
- Davies OG, Williams S, Goldie K. The therapeutic and commercial landscape of stem cell vesicles in regenerative dermatology. J Control Release. 2023 Jan;353:1096-1106.
- Wang C, Wang M, Xu T, et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019 Jan 1;9(1):65-76.