Exosome Isolation Service from Cell Culture Supernatants
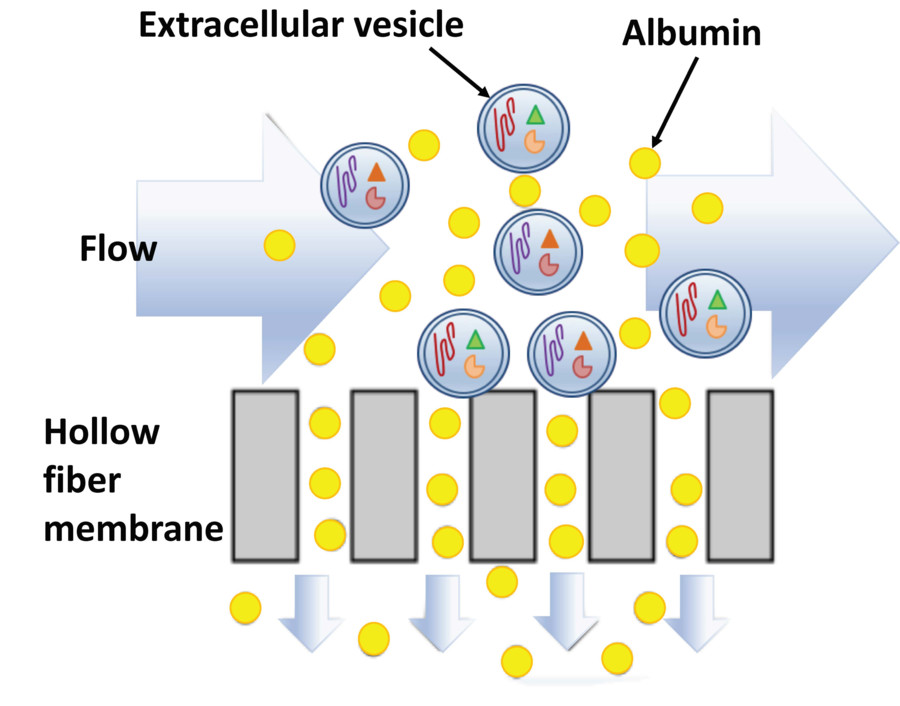
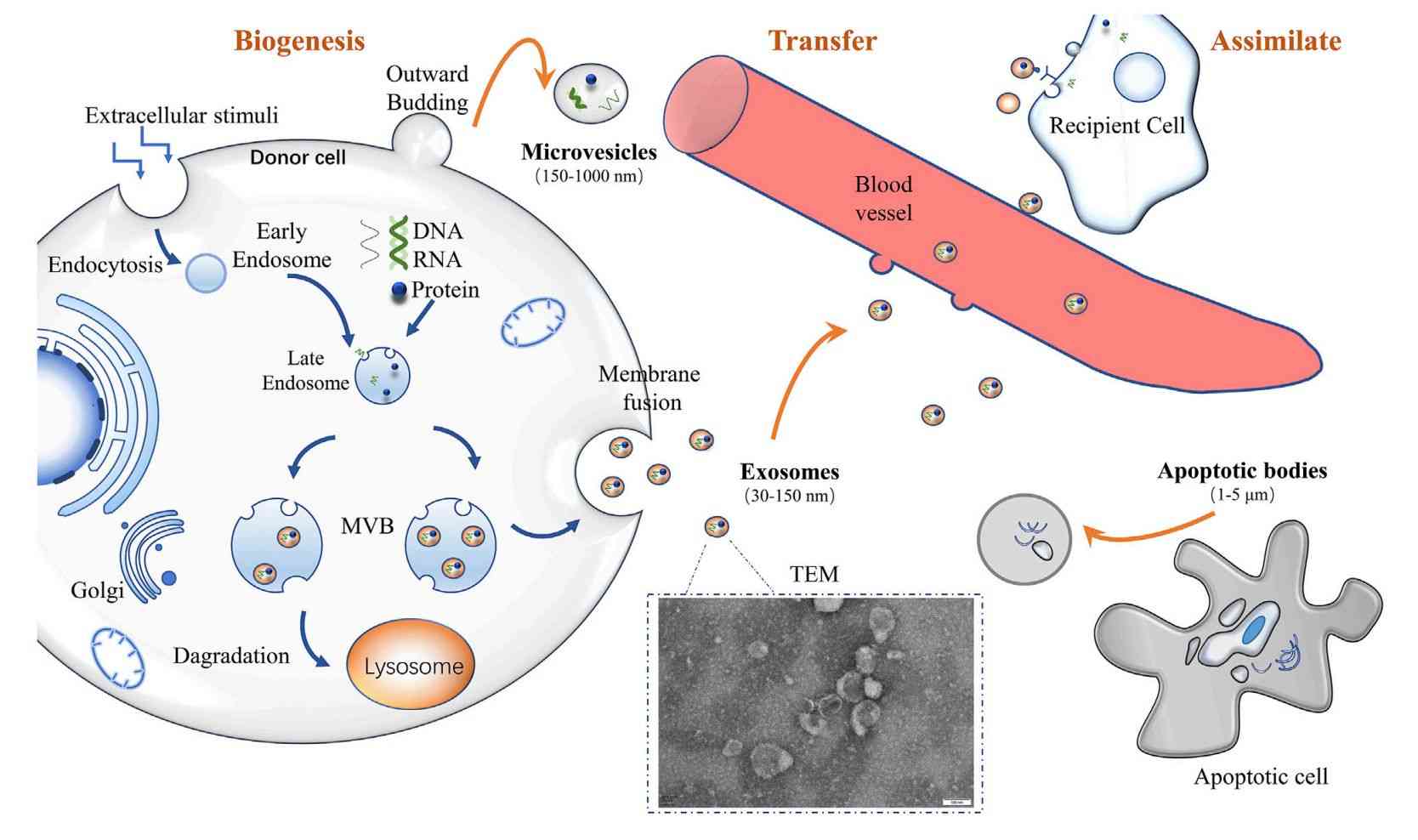
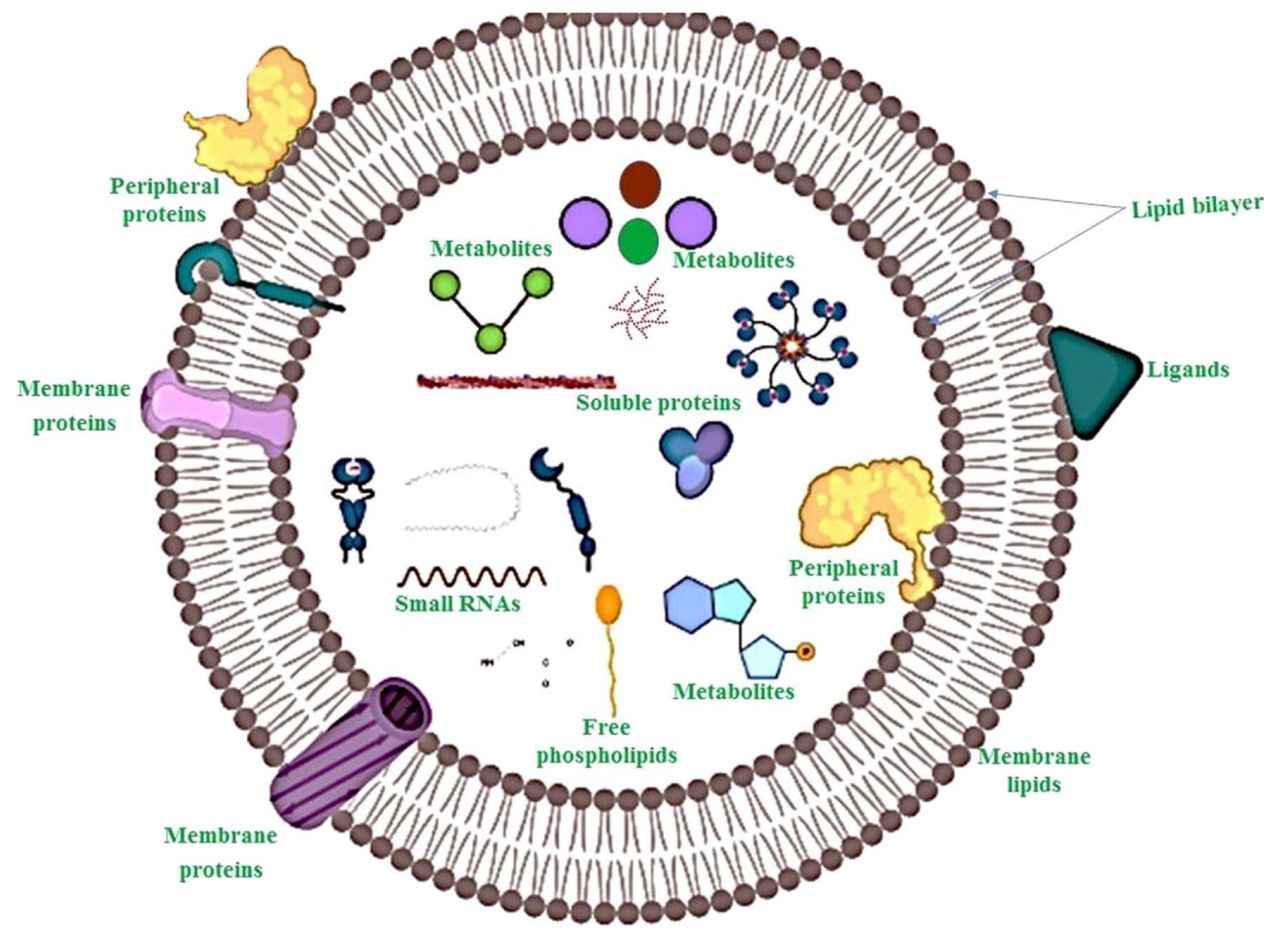
Exosomes are small extracellular vesicles (30 to 150 nm) that play key roles in cell communication, disease progression, and therapeutic delivery. Their value in biomarker discovery and functional research continues to grow. Isolating pure, intact exosomes from cell culture supernatants is technically demanding due to the presence of contaminants such as proteins and cell debris. Many researchers face challenges in achieving consistent purity and yield.
Creative Biostructure offers a dedicated exosome isolation service that addresses these challenges. With proven methodologies and expert support, we provide high-quality exosome preparations suitable for downstream research and development.
Why Isolate Exosomes from Cell Culture Supernatants?
Cell culture supernatants offer a clean, defined source of exosomes—ideal for controlled studies and consistent results. Compared to more complex fluids like plasma or serum, they provide:
- Lower background contamination for clearer analytical results
- Improved reproducibility across experimental batches
- Simpler protocol optimization for method development
- High suitability for in vitro studies and therapeutic vesicle engineering
This makes them the preferred starting material for many exosome research and development pipelines.
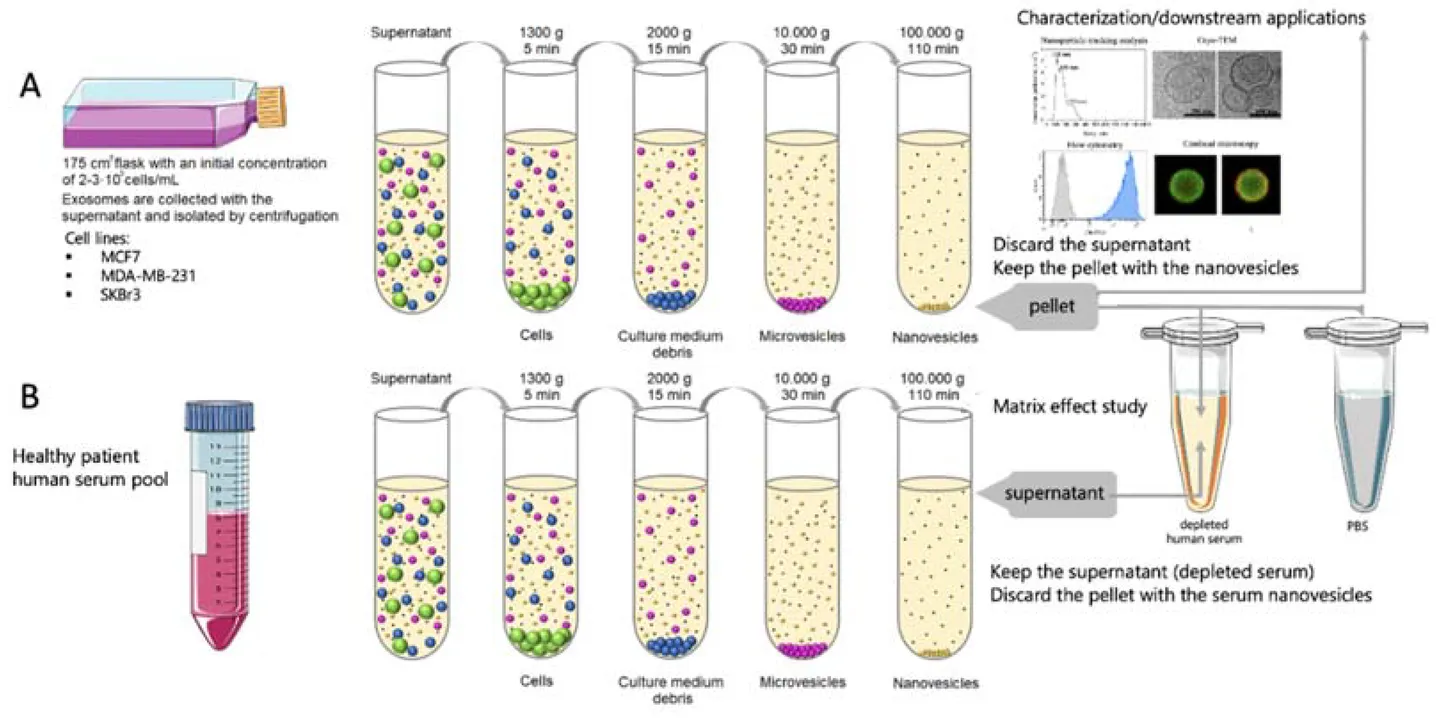
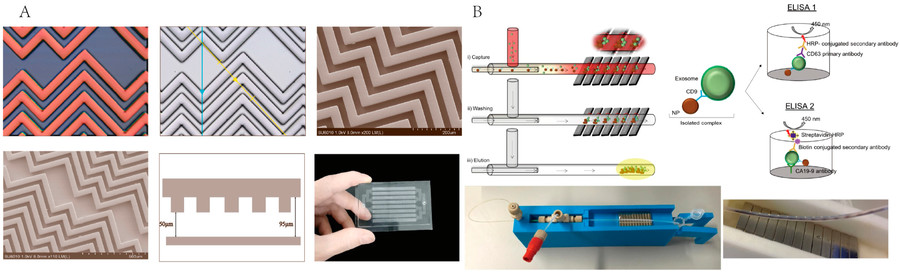
Figure 1. Workflow of Exosome Isolation and Serum Matrix Effect Study. (A) Overview of exosome isolation from breast cancer cell culture supernatant. (B) Preparation of exosome-depleted human serum and spiking with exosomes or PBS to assess matrix effects at varying serum concentrations. (Lima Moura S, et al., 2020)
Exosome Isolation Techniques Offered
Creative Biostructure offers multiple exosome isolation strategies to accommodate different research needs, sample types, and downstream applications. Each method is rigorously validated for yield, purity, and vesicle integrity.
| Technique | Description | Recommended Application Scenarios |
|---|---|---|
| Differential Ultracentrifugation | A classic method based on stepwise centrifugation. Suitable for large volumes but may co-isolate contaminants. Often used for routine studies. | Bulk isolation, signaling studies, general research |
| Density Gradient Ultracentrifugation | Improves purity by separating vesicles based on buoyant density. Ideal for removing proteins and non-vesicular particles. | Proteomics, biomarker discovery, EV subtype separation |
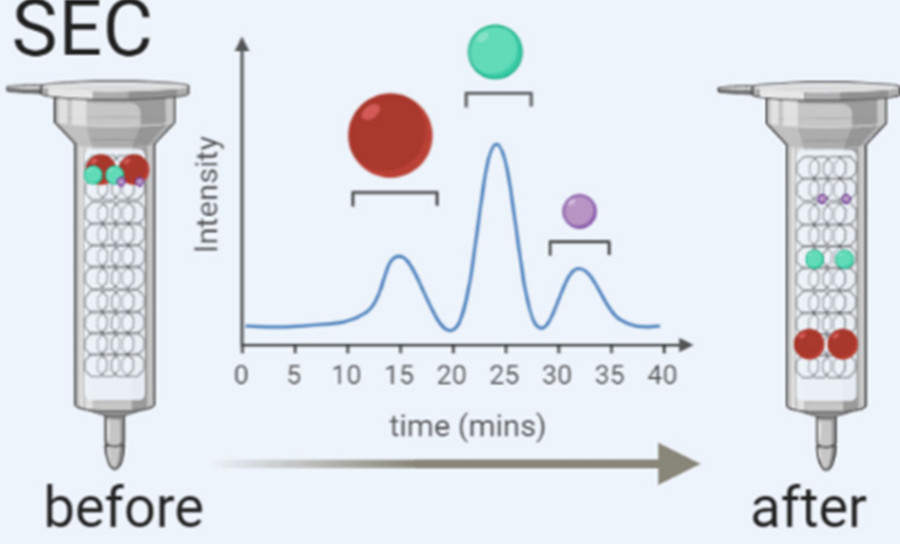
| Size Exclusion Chromatography (SEC) | Column-based separation offering gentle, high-purity exosome recovery without damage. Preserves function and structural integrity. | Functional assays, mass spectrometry, RNA profiling |
| Ultrafiltration (Centrifugal) | Fast concentration using size-selective membranes. Higher recovery and less vesicle loss than pressure-driven systems. | Rapid processing, medium-throughput, pre-enrichment |
| Polymer-Based Precipitation | Convenient, kit-based approach for small volumes. May co-precipitate protein contaminants; follow-up purification recommended. | Pilot studies, RNA analysis, early screening. |
| Immunoaffinity Capture | Magnetic bead- or membrane-based capture targeting surface markers (e.g., CD63, CD81) for selective exosome enrichment. | Targeted isolation, cancer exosomes, biomarker work |
Our multi-platform flexibility allows us to combine techniques (e.g., ultrafiltration + SEC, or gradient + affinity capture) for maximum yield and specificity. Each method is backed by rigorous protocol validation and can be scaled or customized to meet experimental demands.
Tailored Isolation Parameters and Add-On Options
We offer flexible service configurations to align with your experimental needs and sample characteristics. Customization options include:
- Selection of Isolation Method: Choose from ultracentrifugation, SEC, ultrafiltration, or immunoaffinity capture, based on required yield, purity, and downstream application.
- Nuclease Treatment: RNase and DNase treatment available to remove extracellular nucleic acid contaminants for cleaner molecular analysis.
- Downstream Molecular Analysis: Optional profiling of exosomal RNA, proteins, or lipids via NGS, mass spectrometry, or lipidomics platforms, upon request.
- Buffer Choice and Delivery Format: Final exosome products can be resuspended in PBS, HEPES, or custom buffers, and delivered in frozen aliquots or lyophilized form.
Every project begins with a consultation to determine the most appropriate parameters based on your research goals.
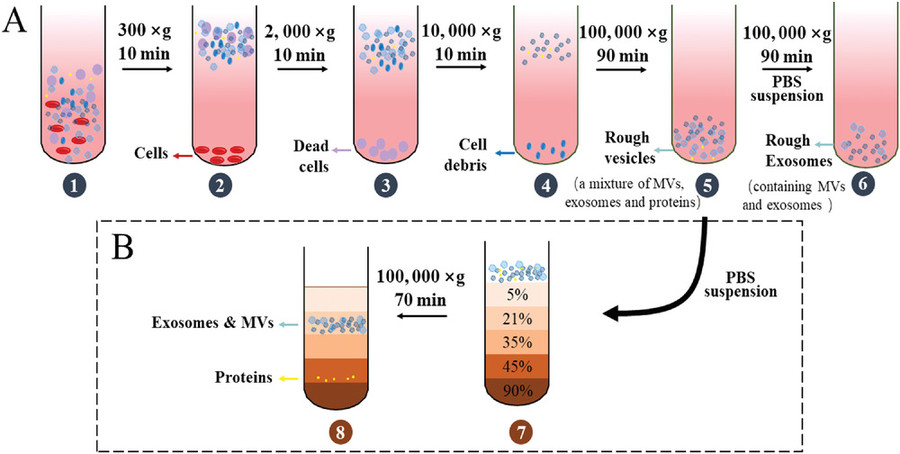
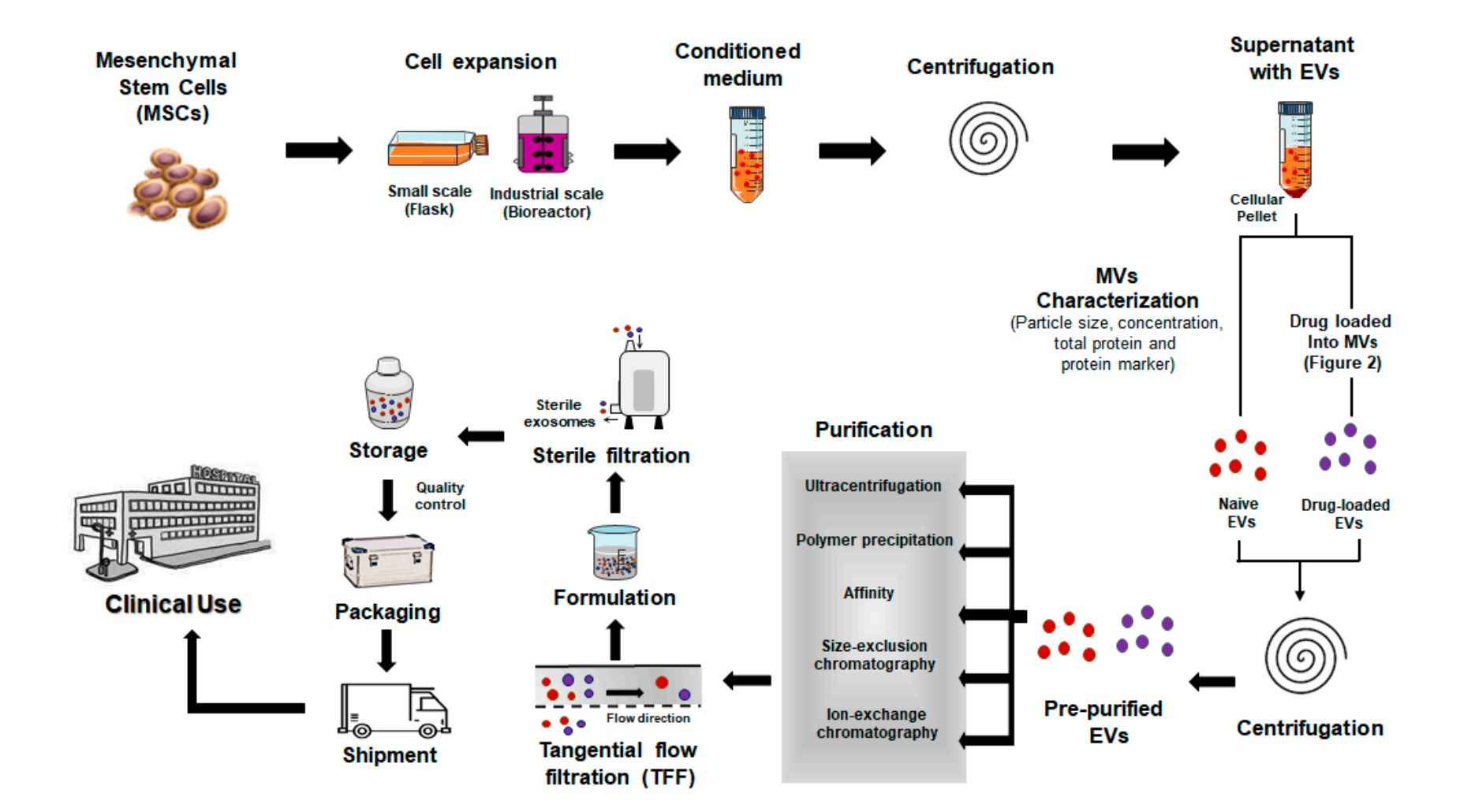
Our Exosome Purification Workflow from Cell Culture Supernatants
We employ a robust and customizable workflow for isolating exosomes from cell culture supernatants, designed to ensure high yield, integrity, and consistency across experimental replicates. Each project is processed under sterile, RNase/DNase-free conditions, with optional steps tailored to downstream applications.
Our standard workflow includes the following key steps:
Pre-clearing of Cellular Contaminants
Conditioned media are subjected to sequential low-speed centrifugation and filtration to remove cells, apoptotic bodies, and large debris, minimizing background interference.
Exosome Enrichment
To align with your research objectives, we select the optimal isolation method, whether ultracentrifugation, size exclusion chromatography, ultrafiltration, or immunoaffinity capture, to concentrate and purify exosomes from the cleared medium.
Concentration and Buffer Exchange
Isolated exosomes are concentrated and re-suspended in sterile, research-grade buffers (e.g., PBS, HEPES), and can be provided in small aliquots or bulk volume depending on your experimental design.
Optional Characterization and Quality Control
For projects requiring analytical validation, we offer optional quality control services including nanoparticle tracking analysis (NTA), Western blotting for exosomal markers (CD63, CD9, CD81), TEM imaging, and particle-to-protein ratio assessment.
We support a wide range of input volumes (from <5 mL to >500 mL) and offer flexible output formats such as frozen liquid aliquots or lyophilized powder. All preparations are documented with detailed protocols and optional technical data sheets.
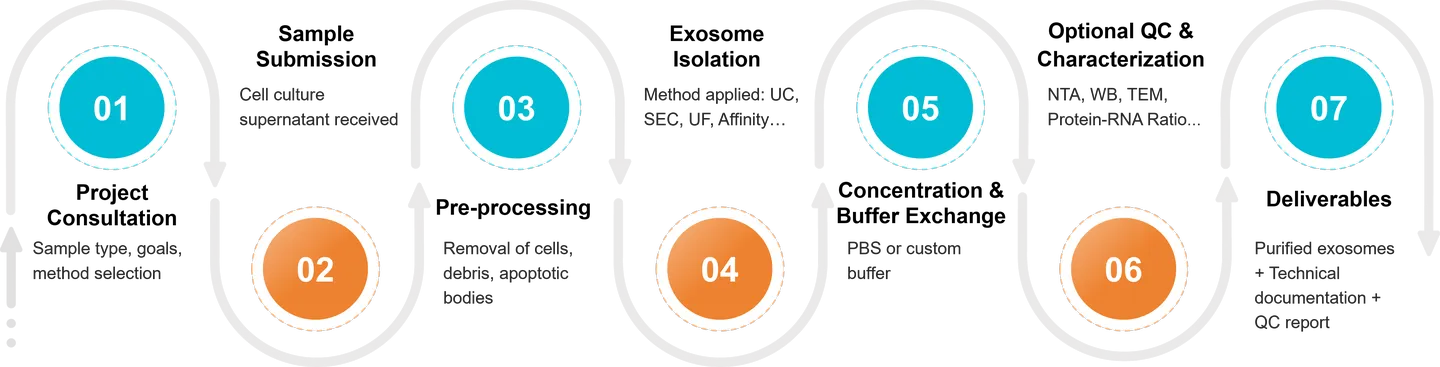
Figure 2. Exosome Isolation and Purification Project Workflow. (Creative Biostructure)
Comprehensive Quality Control
Each batch of isolated exosomes undergoes rigorous characterization using:
- Nanoparticle Tracking Analysis (NTA) for size distribution and concentration
- Transmission Electron Microscopy (TEM) for morphological assessment
- Western Blotting for exosomal markers (CD9, CD63, CD81)
- Purity Assessment via protein-to-particle ratio and co-isolated protein content
Sample Requirements
We accept a wide range of cell culture supernatants, including from adherent and suspension cultures. To ensure optimal exosome yield and quality, we recommend:
- Minimum volume: 10 mL (kit-based) to 50 mL (ultracentrifugation-based); larger volumes welcome for higher yield needs
- Pre-treatment: Cells should be cultured in exosome-depleted or serum-free medium for at least 24-48 hours
- Clarification: Please avoid freeze-thaw cycles; filtered or freshly collected supernatant is preferred
- Common cell sources supported:
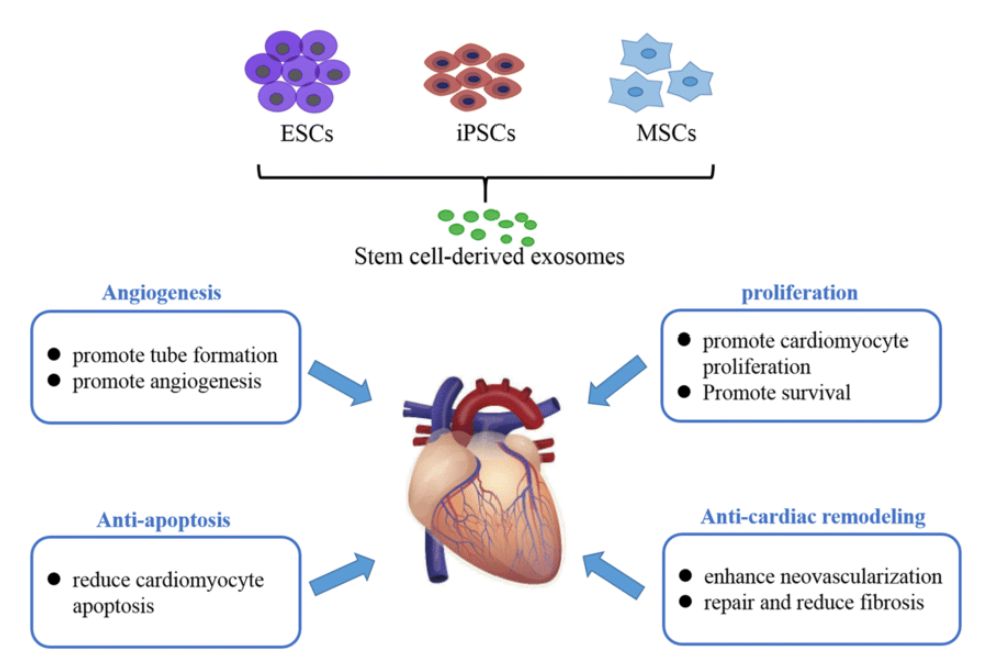
- Mesenchymal stem cells (hMSCs, ADSCs, BMSCs)
- Tumor-derived lines (e.g., MDA-MB-231, HeLa, HCT116, SK-MES-1)
- Immune cells (e.g., Jurkat, THP-1, dendritic cells, NK cells)
- HEK293 and other engineered lines for exosome payload delivery
- iPSCs and neuronal cultures for neurodegenerative disease research
- Endothelial and epithelial cell models
Please contact us for guidance on preconditioning or special sample types.
Deliverables
Upon completion of the service, you will receive:
- Isolated exosomes in sterile PBS or custom buffer (concentrated or lyophilized)
- Yield report indicating particle concentration and total protein (if applicable)
- Optional QC data, including NTA, Western blot, TEM images, or functional assay results
- Technical summary detailing the isolation protocol and handling instructions
Customized data packages and co-branded project reports are available for publication or regulatory support.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.
Case Study
Case: Improved Exosome Yield from MSCs Using One-Step Sucrose Cushion Ultracentrifugation
Background
This study evaluated a modified one-step sucrose cushion ultracentrifugation (SUC) method to enhance the isolation of exosomes from bone marrow– and adipose-derived mesenchymal stem cells (MSCs), compared with traditional differential ultracentrifugation (UC).
Key Findings
- Higher Yield:
BMSCs: 7×10⁹ (SUC) vs. 2×10⁹ particles/mL (UC)
ADSCs: 5.6×10⁹ (SUC) vs. 3.06×10⁹ particles/mL (UC) - Better Vesicle Integrity:
TEM showed intact cup-shaped morphology in SUC-isolated exosomes
UC samples showed fewer and more irregular vesicles - Improved Marker Retention:
CD63⁺ exosomes from BMSCs: 35.5% (SUC) vs. 28.3% (UC)
Stronger Alix and CD63 expression in Western blot for SUC
Conclusion
The one-step SUC method significantly improves exosome recovery and purity, making it a preferred option for MSC-derived exosome production in analytical applications.
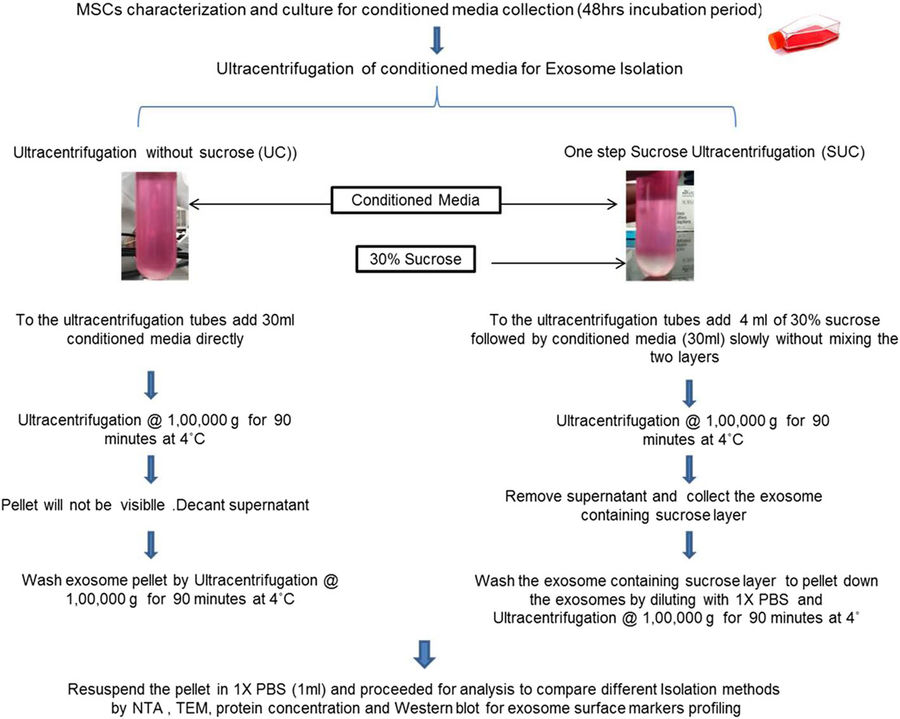
Figure 3. Comparison of Standard and Modified Exosome Isolation Methods. Schematic comparison of direct ultracentrifugation and one-step sucrose cushion ultracentrifugation for isolating exosomes from MSCs. Analytical methods include NTA and TEM, with PBS used as the suspension buffer. (Gupta S, et al., 2018)
At Creative Biostructure, we specialize in tailored exosome isolation services from various cell culture systems, combining high yield with exceptional purity. Whether you're working with stem cells, tumor lines, or engineered cultures, our proven workflows and optional QC ensure data-ready exosome samples. Contact us today to discuss your project and receive a personalized solution.
References
- Lima Moura S, Martì M, Pividori M I. Matrix effect in the isolation of breast cancer-derived nanovesicles by immunomagnetic separation and electrochemical immunosensing—a comparative study. Sensors. 2020, 20(4): 965.
- Gupta S, Rawat S, Arora V, et al. An improvised one-step sucrose cushion ultracentrifugation method for exosome isolation from culture supernatants of mesenchymal stem cells. Stem Cell Research & Therapy. 2018, 9(1): 180.