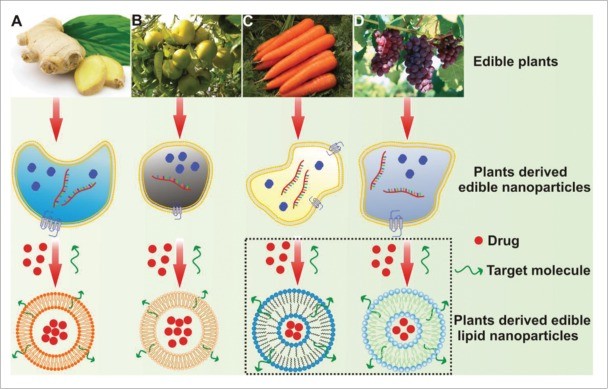
Exosome Stability and Bioavailability Assessment
Developing a commercial exosome product requires more than just biological activity; it requires physical robustness. Can your product survive 12 months on a shelf? Does it withstand freeze-thaw cycles? And most importantly, once ingested, does it actually reach the target tissue in therapeutic concentrations?
We provide comprehensive Exosome Stability and Bioavailability Assessment solutions. We combine pharmaceutical-grade stability testing (ICH guidelines) with advanced Pharmacokinetic (PK) studies to provide the definitive data package needed for product labeling, expiration dating, and efficacy substantiation.
Two Critical Hurdles for Commercialization
To move from the lab to the market, you must answer two questions:
- Shelf-Life Stability: Liquid exosomes are unstable. They aggregate, leak cargo, and lose surface markers over time. Developing a stable formulation (e.g., lyophilized powder) and proving its longevity via accelerated aging tests is mandatory for retail products.
- Systemic Bioavailability: "Oral delivery" is meaningless if the uptake is negligible. You need to quantify the absolute bioavailability (%)—the fraction of the oral dose that actually enters the blood circulation compared to intravenous injection.
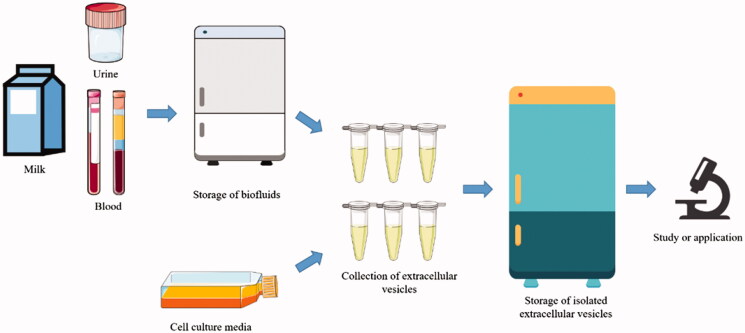 Figure 1. Process illustration for biofluid sample storage and extracellular vesicle collection for study or application. (Yuan F, et al., 2021)
Figure 1. Process illustration for biofluid sample storage and extracellular vesicle collection for study or application. (Yuan F, et al., 2021)
Our Assessment Workflow
We offer a dual-track evaluation pipeline covering both storage quality and in vivo performance.
| Assessment Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Storage Stability Testing | Real-Time & Accelerated: We store exosomes at various temperatures (-80°C, 4°C, 25°C) and formulate them with cryoprotectants (Trehalose). We monitor Particle Size (NTA) and Zeta Potential monthly to detect aggregation or fusion. | Exosome Particle Size and Concentration Analysis |
| Cargo Integrity Check | Leakage Analysis: A stable vesicle must retain its payload. We use RNase Protection Assays to verify that miRNAs remain encapsulated and protected inside the exosome, rather than leaking into the buffer during storage. | Exosome Identification |
| Pharmacokinetics (PK) | Blood Concentration Profiles: We administer labeled exosomes to animals and collect serial blood samples over 24-48 hours. We calculate Cmax (peak concentration), Tmax (time to peak), and AUC (total exposure) to quantify absorption. | In Vivo Exosome Functional Assays |
| Biodistribution Imaging | Tissue Mapping: Where do they go? We use IVIS Fluorescence Imaging or radioactive tracing to visualize the accumulation of exosomes in the liver, brain, bone, or tumor, proving tissue-specific targeting. | Exosome Tracing and Tracking |
Core Technologies for Quality & PK
We utilize pharmaceutical industry standards to validate your product.
Lyophilization Stability Optimization
Powder Formulation: Liquid storage is logistically difficult. We test and optimize Freeze-Drying Cycles using various bulking agents (Mannitol/Glycine). We use Scanning Electron Microscopy (SEM) to verify that the rehydrated exosomes retain their original spherical morphology and are not destroyed by ice crystal formation.
Radioactive/Fluorescent Tracing
The PK Gold Standard: To track exosomes in vivo, we label them with near-infrared dyes (DiR) or radioisotopes (I-125). This allows for highly sensitive, non-invasive tracking of the exosome's journey from the gut to systemic organs, providing quantitative biodistribution data that regulatory agencies trust.
Half-Life Determination
Circulation Time: How long do they last in the blood? We measure the Circulation Half-Life (t1/2). This is critical for determining dosing frequency. We can also modify the exosome surface (e.g., PEGylation or CD47 enrichment) to reduce clearance by macrophages and extend therapeutic action.
Application Spotlight: Lyophilized Milk Exosomes Retain Bioactivity
This analysis highlights how proper formulation can extend shelf-life from days to months without compromising biological function.
Featured Technologies:
- Lyophilization Optimization
- Long-term Stability Testing
Literature Interpretation:
Bovine milk exosomes are a promising therapeutic, but they require -80°C storage, which complicates logistics. Researchers developed a specialized lyophilization (freeze-drying) protocol for milk exosomes. They tested stability under accelerated aging conditions. The study showed that the lyophilized milk exosome powder remained stable at room temperature for at least 3 months and at 4°C for over 15 months. Crucially, after rehydration, the exosomes retained their original particle size, protein markers (CD63, TSG101), and cellular uptake efficiency. This demonstrates that with the right formulation strategy, exosome products can achieve the shelf-life stability required for commercial pharmaceutical or nutraceutical supply chains.
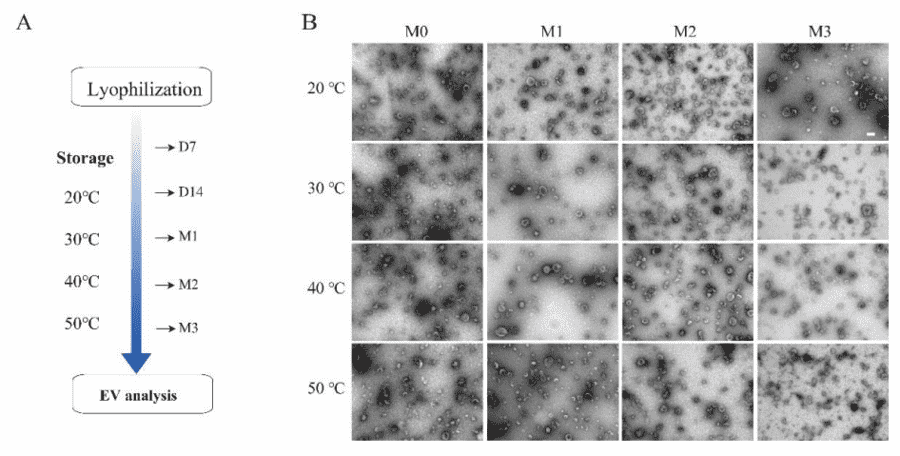 Figure 2. Stability assessment of lyophilized bovine milk-derived exosomes (MK-Exo) under various storage conditions over time. (a) Storage temperatures and time points for analysis. (b) Morphological analysis of MK-Exo at different temperatures and durations. (Lu L, et al., 2024)
Figure 2. Stability assessment of lyophilized bovine milk-derived exosomes (MK-Exo) under various storage conditions over time. (a) Storage temperatures and time points for analysis. (b) Morphological analysis of MK-Exo at different temperatures and durations. (Lu L, et al., 2024)
Start Your Validation Project
Ensure your product is shelf-stable and biologically available before market launch.
How It Works: Our Project Pathway
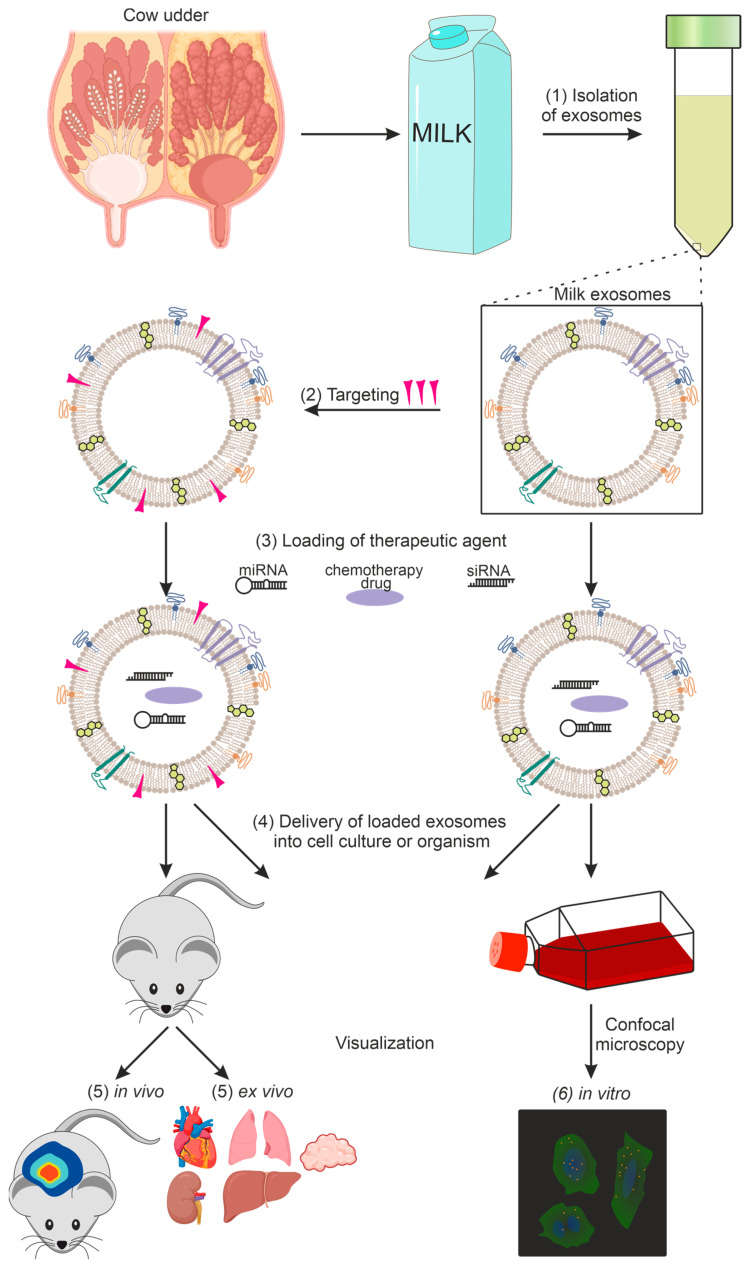
 Figure 3. Our integrated workflow for validating the storage stability and systemic bioavailability of commercial exosome formulations. (Creative Biostructure)
Figure 3. Our integrated workflow for validating the storage stability and systemic bioavailability of commercial exosome formulations. (Creative Biostructure)
Ready to confirm the quality and performance of your exosome product? Our QA and pharmacology teams are available to design a stability and PK study plan. Contact us today to discuss your project.
References
- RYuan F, Li YM, Wang Z. Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. 2021 Dec;28(1):1501-1509.
- Lu L, Han C, Wang M, et al. Assessment of bovine milk exosome preparation and lyophilized powder stability. J Extracell Biol. 2024 Nov 15;3(11):e70009.