Cosmetic Exosome Safety and Efficacy Testing
In the competitive beauty market, "miracle ingredients" are viewed with skepticism. Consumers, retailers, and regulators demand proof. Whether you are launching a post-procedure repair serum or an anti-aging cream, ensuring biological safety and generating robust efficacy data are the prerequisites for market entry.
We provide comprehensive Cosmetic Exosome Safety and Efficacy Testing solutions. We offer a complete testing ecosystem tailored for biological ingredients—from Endotoxin (LAL) screening and Cytotoxicity profiles to advanced 3D Reconstructed Human Skin models. We help you generate the rigorous "Before & After" biological data needed to support high-value claims like "Collagen Boosting," "Soothing," and "Barrier Repair."
The Necessity of Rigorous Testing
Exosomes are biological entities, not simple chemicals. Standard cosmetic testing protocols often require adaptation to accurately assess their safety and function.
- Regulatory Compliance: Ensuring your exosome ingredient is free from cell/tissue residues, viral contaminants, and endotoxins is critical to meeting FDA, EU, and INCI safety standards.
- Claim Substantiation: To legally claim "Anti-Aging" or "Whitening," you need quantitative data. Visual improvement is not enough; molecular proof (e.g., upregulated Collagen I gene expression) provides the necessary scientific backing.
- Alternative to Animal Testing: The industry has moved to cruelty-free testing. Our 3D Reconstructed Human Epidermis (RHE) models provide human-relevant data without animal experimentation.
- Batch Consistency: Biologicals vary. Routine potency testing ensures that every production lot delivers the same biological effect, protecting your brand reputation.
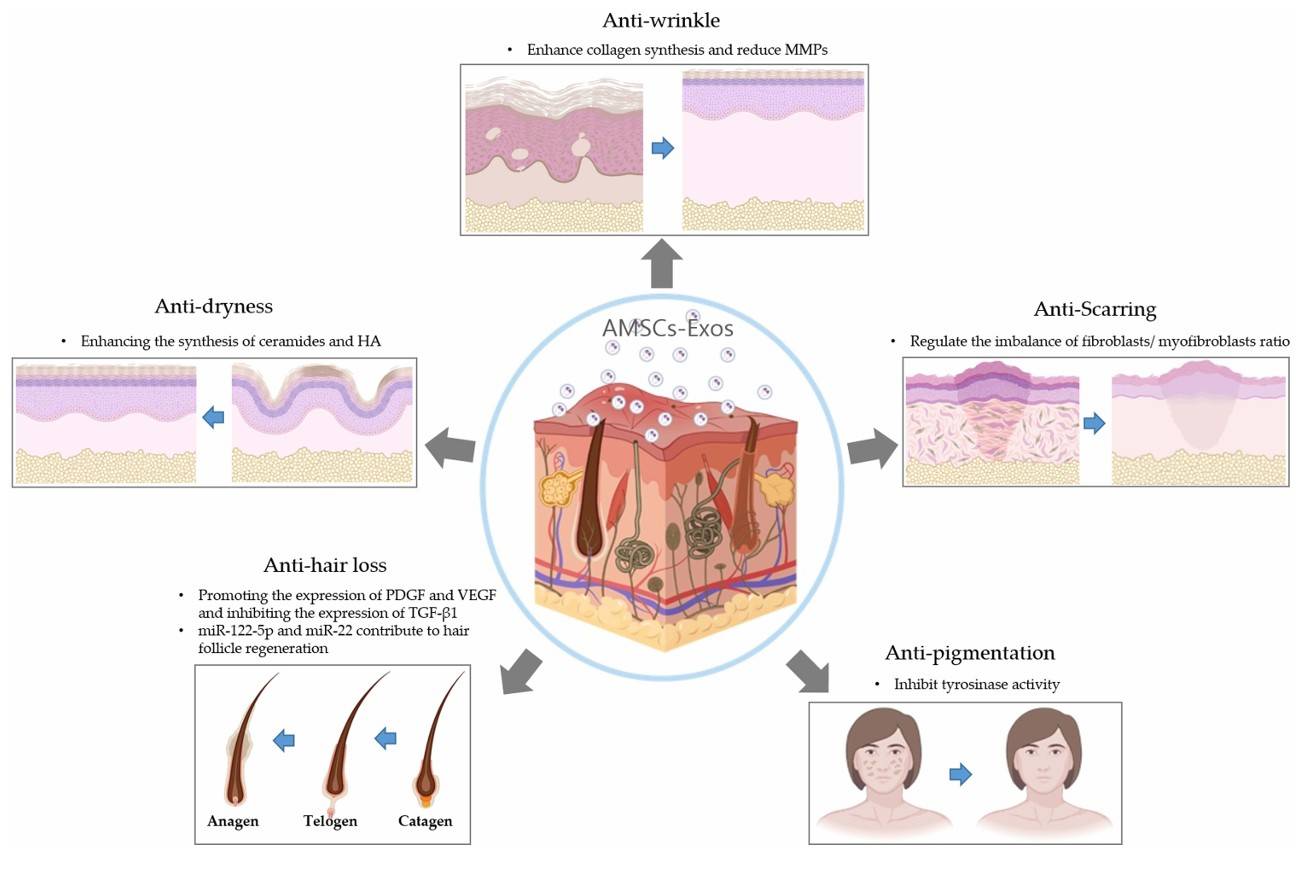
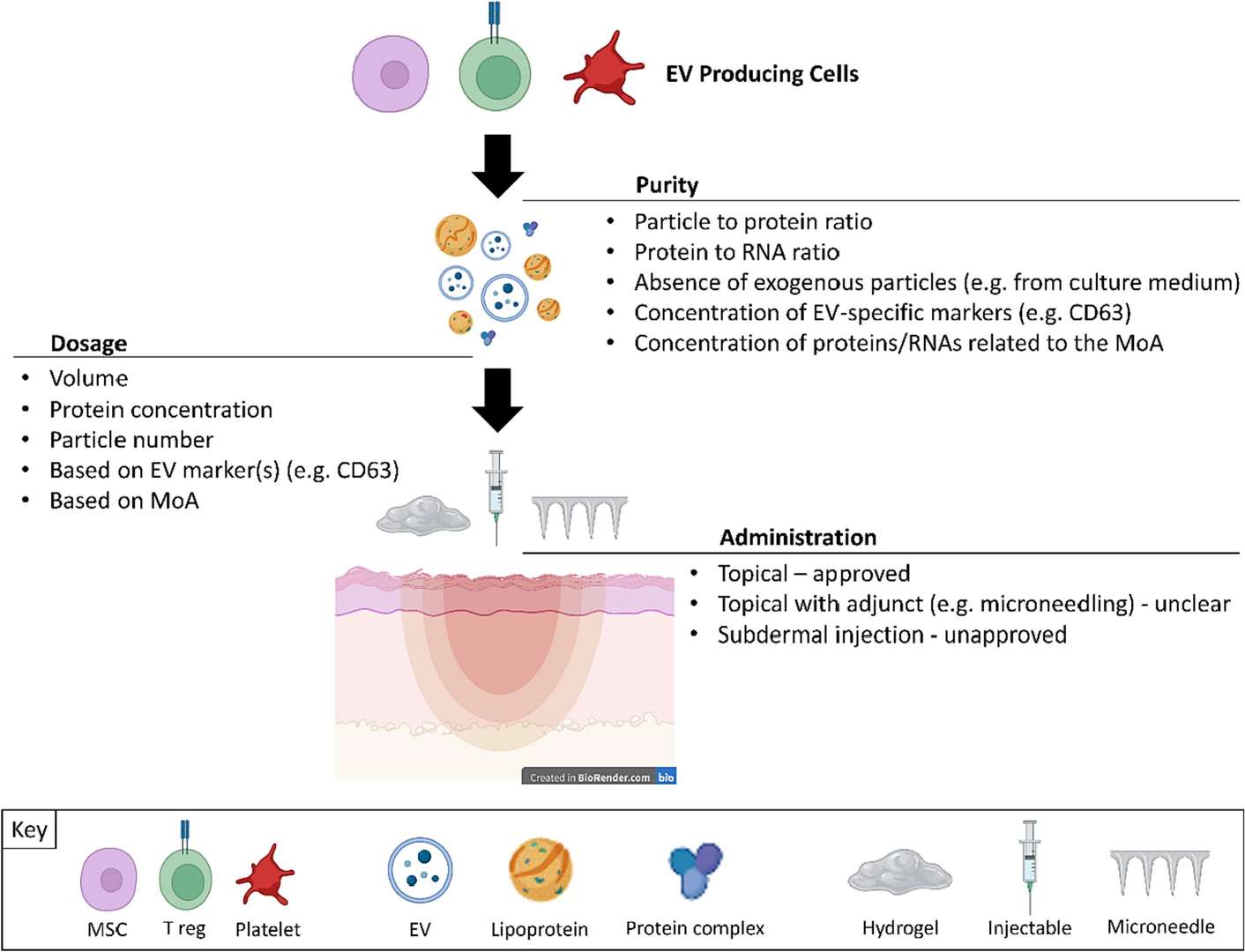
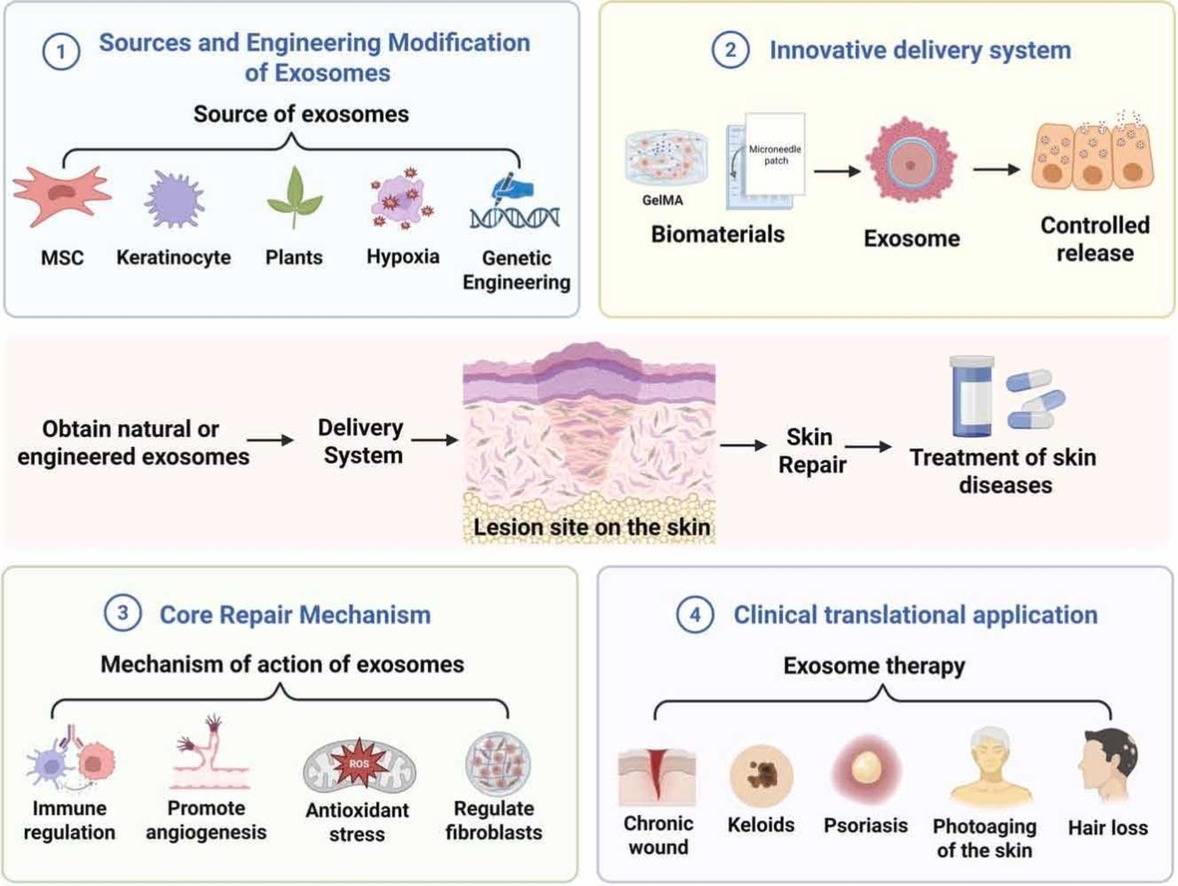 Figure 1. Overview of exosome mechanisms and applications in skin repair and regeneration across various dermatological conditions. (Deng T, et al., 2025)
Figure 1. Overview of exosome mechanisms and applications in skin repair and regeneration across various dermatological conditions. (Deng T, et al., 2025)
Our Tiered Validation Workflow
We utilize a systematic, phased approach to generate the data package required for regulatory filing and marketing substantiation.
| Testing Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Contaminant Screening (Safety) | Purity Verification: Before efficacy testing, we screen for "invisible" contaminants. We perform Chromogenic LAL Assays for endotoxins and qPCR for residual host cell DNA, ensuring the raw ingredient meets safety specs (<0.1 EU/mL). | Exosome Endotoxin Detection, Exosomal Residual DNA/RNA Quantification |
| Tolerance & Cytotoxicity | Defining Safe Limits: We determine the non-toxic concentration range using MTT/CCK-8 assays on human keratinocytes. We also conduct In Vitro Irritation tests (e.g., Het-CAM or RHE viability) to predict potential skin reactions. | Exosome Cellular Functional Assays, Exosome Contaminant Detection |
| Bioactivity & Mechanism | Molecular Proof: We prove how it works. We treat cells and quantify the upregulation of Collagen I/III & Elastin genes (RT-qPCR) or the inhibition of Tyrosinase activity (Enzymatic assay), providing mechanistic evidence for your claims. | Exosomal mRNA Sequencing, Exosome Characterization by Western Blotting |
| 3D Tissue Modeling | Visual Substantiation: We move beyond monolayers to 3D Reconstructed Human Skin. We apply the final formulation topically and use H&E Histology to visually demonstrate epidermal thickening and barrier reinforcement. | Exosome 3D Skin Model Assays, Exosome Morphology Analysis |
Core Technologies for Claim Support
We deploy specific assays to generate the marketing-ready data graphs you need.
3D Reconstructed Human Skin Models
The "Cruelty-Free" Gold Standard: Simple cell cultures cannot show "barrier repair." We use 3D organotypic skin models (like full-thickness equivalents). We apply your exosome formulation topically and analyze tissue morphology, lipid organization, and hydration markers (Filaggrin/Loricrin), providing powerful visual evidence of skin reconstruction that mimics real human application.
Endotoxin & Sterility Testing
Ensuring "Sensitive Skin" Safety: Exosomes derived from cells can carry bacterial endotoxins (LPS) if not processed correctly, causing redness and irritation. We utilize high-sensitivity Chromogenic LAL Assays to certify that your ingredient is "Low Endotoxin," a critical specification for products designed for post-procedure or sensitive skin care.
Anti-Melanogenesis Assays
Whitening Data: For brightening claims, we use melanocyte co-cultures. We quantify the inhibition of Tyrosinase activity and the reduction of total melanin content after exosome treatment. We also track the downregulation of melanogenic transcription factors (MITF), proving the biological mechanism of your whitening effect.
Application Spotlight: Restoring Skin Barrier via De Novo Ceramide Synthesis
This analysis demonstrates how molecular assays can substantiate high-value claims like "barrier repair" and "lipid boosting" by tracking specific enzymatic pathways.
Featured Technologies:
- Efficacy Assays (Gene/Protein Expression)
- Lipidomic Profiling
Literature Interpretation:
Atopic dermatitis and sensitive skin are characterized by a critical deficiency in ceramides, the essential "mortar" of the skin barrier. Researchers investigated the therapeutic potential of Adipose MSC Exosomes on compromised skin cells. Utilizing lipidomic analysis and RT-qPCR, the study revealed that the exosomes did not merely coat the skin; they actively upregulated key enzymes (SPTLC, CerS) responsible for de novo lipid synthesis. This biological activation led to a significant increase in endogenous ceramide production and the restoration of barrier function, quantified by reduced Transepidermal Water Loss (TEWL) and improved hydration. These findings provide molecular-level evidence that exosomes can reactivate the skin's own barrier-repairing machinery.
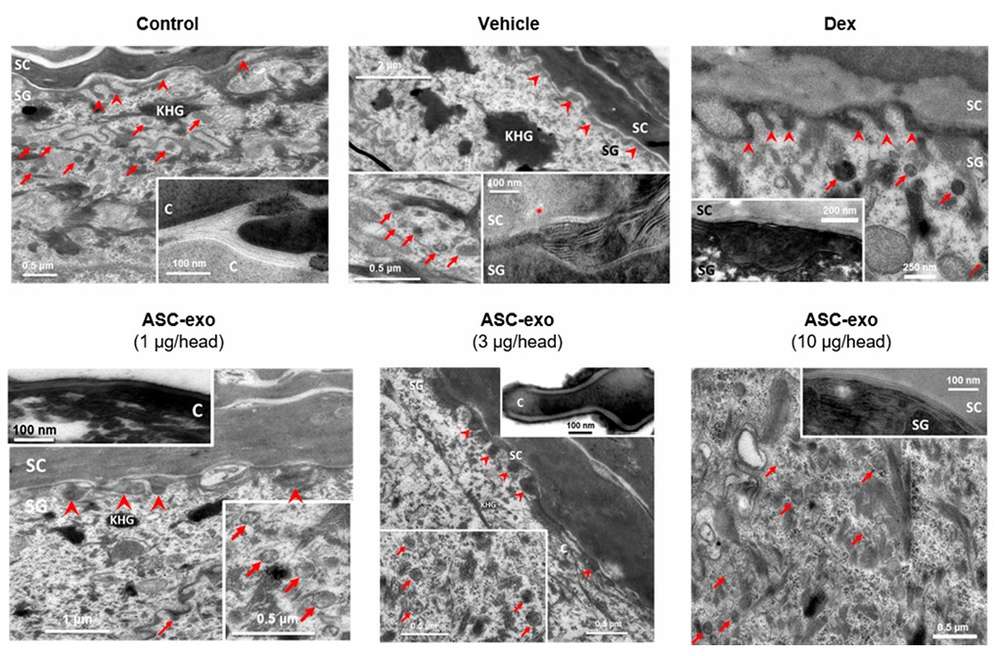 Figure 2. ASC-exosomes promote lamellar body abundance in epidermal cells. High-magnification views reveal increased presence at the SG-SC interface post-treatment compared to controls. (Shin KO, et al., 2020)
Figure 2. ASC-exosomes promote lamellar body abundance in epidermal cells. High-magnification views reveal increased presence at the SG-SC interface post-treatment compared to controls. (Shin KO, et al., 2020)
Start Your Testing Project
Ensure your product is safe, compliant, and proven to work before it hits the market.
How It Works: Our Project Pathway
 Figure 3. Our rigorous testing workflow for validating the safety and biological efficacy of exosome ingredients using advanced in vitro and 3D tissue models. (Creative Biostructure)
Figure 3. Our rigorous testing workflow for validating the safety and biological efficacy of exosome ingredients using advanced in vitro and 3D tissue models. (Creative Biostructure)
Ready to back your skincare claims with rigorous science? Our testing team is available for a free consultation to design your safety and efficacy study plan. Contact us today to discuss your project.
References
- Deng T, Zhang Y, Yao Y, et al. Exosome therapeutics: A paradigm shift in skin repair through multidimensional immunomodulation and biomaterial-driven delivery. Biomed Pharmacother. 2025 Nov 29;193:118830.
- Shin KO, Ha DH, Kim JO, et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells. 2020 Mar 10;9(3):680.