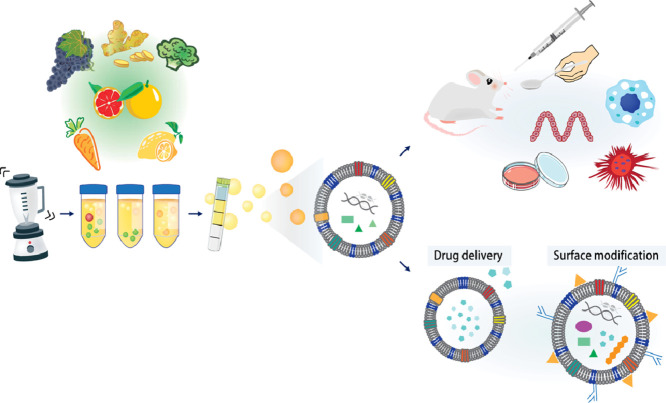
Kidney and Liver Disease Exosome Solutions
Kidney and liver diseases are often silent killers, detected only when significant damage has occurred. Traditional biopsies are invasive and risky.
We provide end-to-end kidney and liver exosome solutions. We enable you to utilize urinary exosomes as a "liquid kidney biopsy" and circulating exosomes to monitor hepatic health. Whether you are investigating exosomes chronic kidney disease (CKD) progression, liver regeneration, or the complex inter-organ communication that drives metabolic pathology, our platform provides the specialized isolation and tracking tools you need.
The Role of Exosomes in Renal and Hepatic Health
Why are exosomes critical for studying these organs? They are the active messengers in systemic homeostasis and disease progression.
- The "Liquid Kidney Biopsy": Urinary exosomes in kidney diseases originate from every part of the nephron (glomerulus, tubule). They carry specific transporters (like ENaC, AQP2) and biomarkers that reflect real-time renal function, offering a powerful tool for monitoring CKD or kidney dialysis efficacy.
- The Liver as a Hub: The liver is the central dock for systemic signals. Adipocyte exosome liver crosstalk drives fatty liver disease (NASH), while tumor-derived exosomes often target the liver to prepare the soil for metastasis (pre-metastatic niche).
- Regeneration & Repair: Exosomes liver regeneration studies show that stem cell exosomes can accelerate hepatic repair after injury. Similarly, exosomes and kidney repair research focuses on reducing renal fibrosis.
- Systemic Tracking: Understanding these diseases requires seeing where the signals go. We utilize live tracking of inter-organ communication by endogenous exosomes in vivo to map how exosomes travel from the gut or pancreas to the liver.
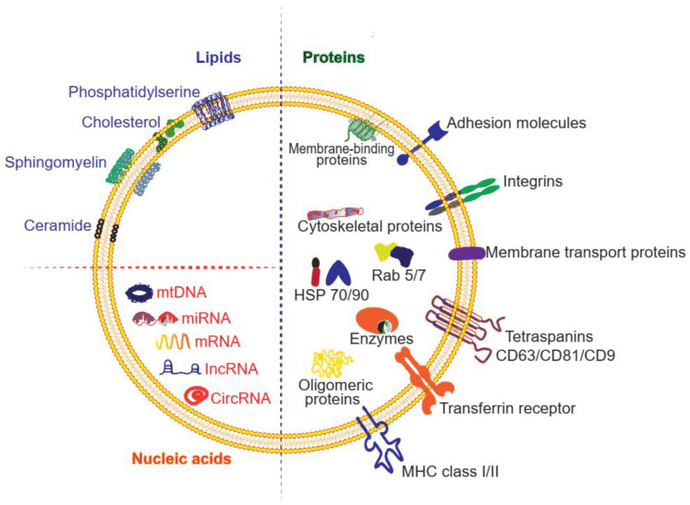
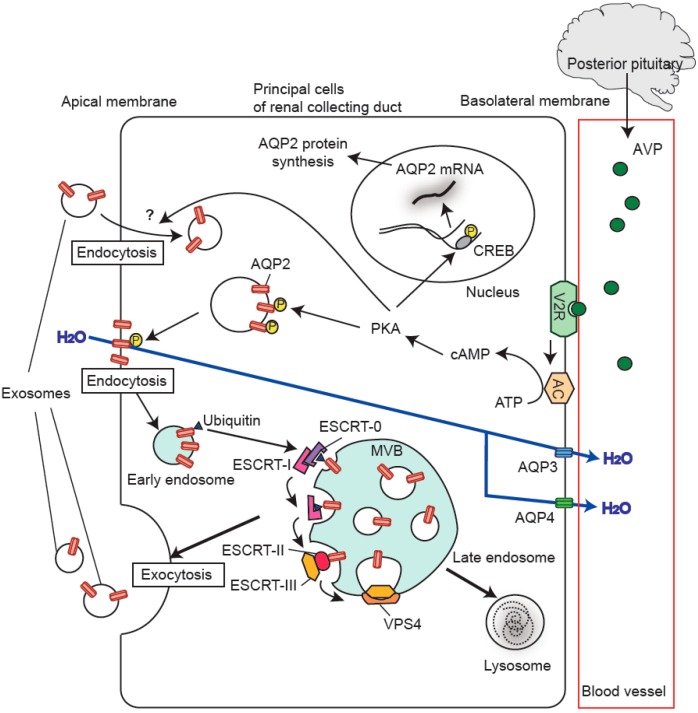 Figure 1. Exosomal AQP2 genesis in renal cells. Vasopressin activates V2R, leading to AQP2 phosphorylation and increased expression via PKA and CREB. Ubiquitinated AQP2 forms exosomes through ESCRT complexes. (Wen H, et al., 2021)
Figure 1. Exosomal AQP2 genesis in renal cells. Vasopressin activates V2R, leading to AQP2 phosphorylation and increased expression via PKA and CREB. Ubiquitinated AQP2 forms exosomes through ESCRT complexes. (Wen H, et al., 2021)
Our Integrated Organ-Specific Workflow
Kidney (Urine) and Liver (Plasma/Tissue) samples present unique challenges. Our workflow is optimized for these matrices.
| Service Pillar | Key Services & Technologies We Provide |
|---|---|
| Urine & Plasma Isolation | Matrix-Specific Protocols: For the kidney, we use specialized TFF/SEC to isolate urinary exosomes while removing Tamm-Horsfall protein (THP) which traps vesicles. For the liver, we isolate from plasma or tissue, utilizing liver exosome electron microscope (TEM) imaging to verify morphology. |
| Organ-Specific Profiling | Biomarker Discovery: We profile cargo specific to organ function. This includes identifying exosomal biomarkers for kidney disease (e.g., Nephrin, Podocin) or liver damage (e.g., albumin-mRNA, specific miRNAs). |
| Biodistribution Tracking | Visualizing Signaling: We use lipophilic dyes (DIR/DIO) or genetic reporters (CD63-GFP) for biodistribution ev exosome liver studies. We track how injected exosomes accumulate in the liver or kidneys in vivo. |
| Functional Disease Models | Mechanism Validation: We test efficacy in relevant models, such as proximal tubule cells (for exosomes regulate ENaC in kidney) or hepatic stellate cells (for liver fibrosis). |
Organ-Specific Applications We Support
Our platform is tailored to investigate the unique pathologies of the renal and hepatic systems.
Kidney Disease Exosome Profiling
We support the discovery of non-invasive markers for Chronic Kidney Disease (CKD), AKI, and Nephrotic Syndrome. By analyzing urinary exosomes, we help you monitor the loss of specific podocyte proteins or changes in sodium transporters (ENaC), potentially serving as indicators for patients undergoing kidney dialysis to assess residual renal function.
Liver Disease & Regeneration Solutions
From NAFLD/NASH to fibrosis, we analyze how liver exosomes modulate inflammation and stellate cell activation. We also support exosome therapy for liver damage, testing the ability of MSC exosomes to promote hepatocyte proliferation and inhibit fibrogenesis.
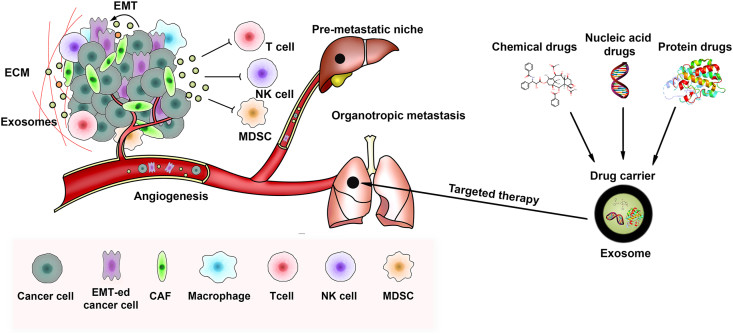
Metastatic Niche Research (Pancreas-to-Liver)
A critical area of cancer research is how pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. We provide specific models to isolate tumor exosomes and track their uptake by Kupffer cells in the liver, helping you decode the mechanisms of organotropic metastasis (cancer derived exosome decm liver).
Advantages of Our Kidney & Liver Platform
Researching excretory and metabolic organs requires overcoming specific biological barriers. We have optimized our platform to solve these matrix-specific challenges through our specialized service modules.
Specialized Urine Isolation via TFF
Urine contains Tamm-Horsfall Protein (THP) which traps exosomes, leading to massive yield loss in standard kits. We solve this with Tangential Flow Filtration (TFF). By combining TFF with chemical depolymerization, we successfully process large volumes of urine to recover the full spectrum of kidney exosomes—from glomerulus to collecting duct—ensuring your biomarkers are not lost during isolation.
Liver Exosome Electron Microscopy
Liver samples are often high in lipids and protein aggregates, which can be mistaken for exosomes. We provide definitive proof of isolation quality through TEM Characterization. We deliver high-resolution liver exosome electron microscope images that allow you to visually distinguish true vesicles from contaminants, providing the "Gold Standard" validation required by top-tier journals.
In Vivo Live Tracking Services
Proving "Inter-Organ Crosstalk" (e.g., Gut-Liver axis) requires visualizing the journey. We enable this through Exosome Tracing and Tracking Service. Using lipophilic dyes (DiR) or CD63-GFP reporters, we perform live tracking of inter-organ communication by endogenous exosomes in vivo. This allows you to quantify exactly how much of your sample accumulates in the liver versus the kidney.
Integrated Multi-Omics for Fluid Biopsy
Kidney and liver biopsies are invasive; liquid biopsies are the future. Our Multi-Omics Integration is optimized to extract both RNA and Protein from the same low-volume biofluid sample. This maximizes the data generated from your urinary exosomes, allowing you to correlate miRNA changes with protein markers to discover robust exosomal biomarkers for kidney disease.
Application Spotlight: Pancreatic Exosomes Prime the Liver for Metastasis
This analysis highlights the role of exosomes in inter-organ communication, specifically how they establish a permissive microenvironment for cancer spread.
Featured Technologies:
- Tumor Exosome Isolation
- Biodistribution Tracking (In Vivo)
Literature Interpretation:
This seminal paper established the concept of the "pre-metastatic niche" in the liver. Researchers investigated why pancreatic cancer so often spreads to the liver. They found that pancreatic cancer exosomes traveled to the liver and were taken up by Kupffer cells. These exosomes carried Macrophage Migration Inhibitory Factor (MIF), which induced liver fibrosis and inflammation before the cancer cells even arrived. This process, known as initiating pre-metastatic niche formation, creates a hospitable environment for metastasis.
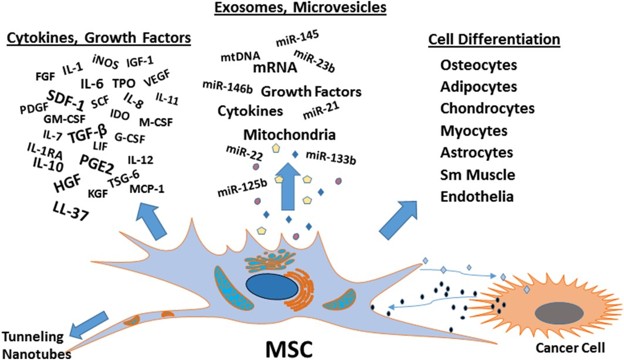
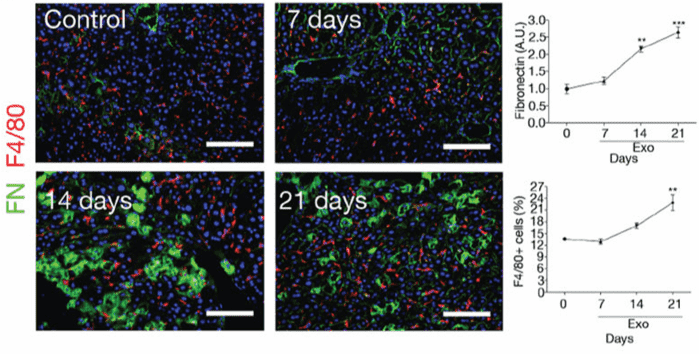 Figure 2. PAN02 exosomes induce fibronectin expression and F4/80+ cell frequency in mouse liver. Immunofluorescence quantification of FN expression (A.U.) and F4/80+ cell frequency over time (n=4 mice). Scale bars, 100μm. (Costa-Silva B, et al., 2015)
Figure 2. PAN02 exosomes induce fibronectin expression and F4/80+ cell frequency in mouse liver. Immunofluorescence quantification of FN expression (A.U.) and F4/80+ cell frequency over time (n=4 mice). Scale bars, 100μm. (Costa-Silva B, et al., 2015)
Start Your Kidney & Liver Project
We make getting started straightforward. Our process is designed to be collaborative and transparent.
How It Works: Our Project Pathway
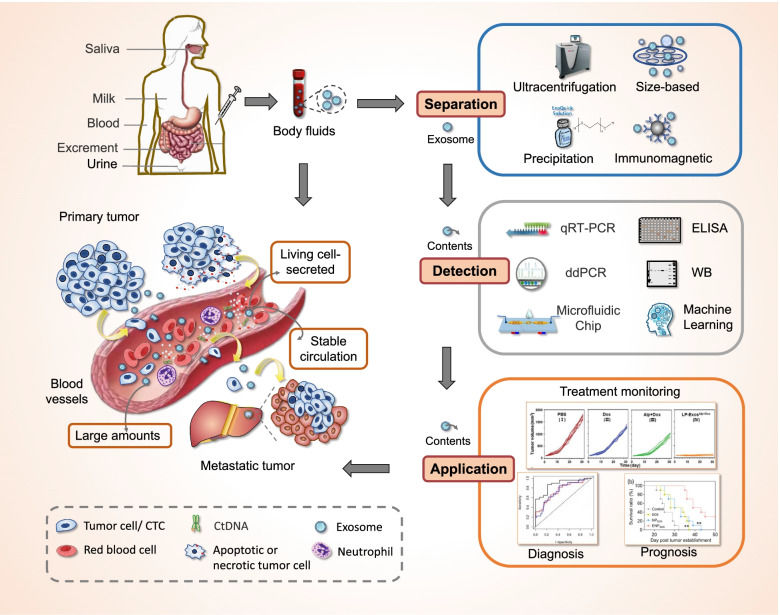
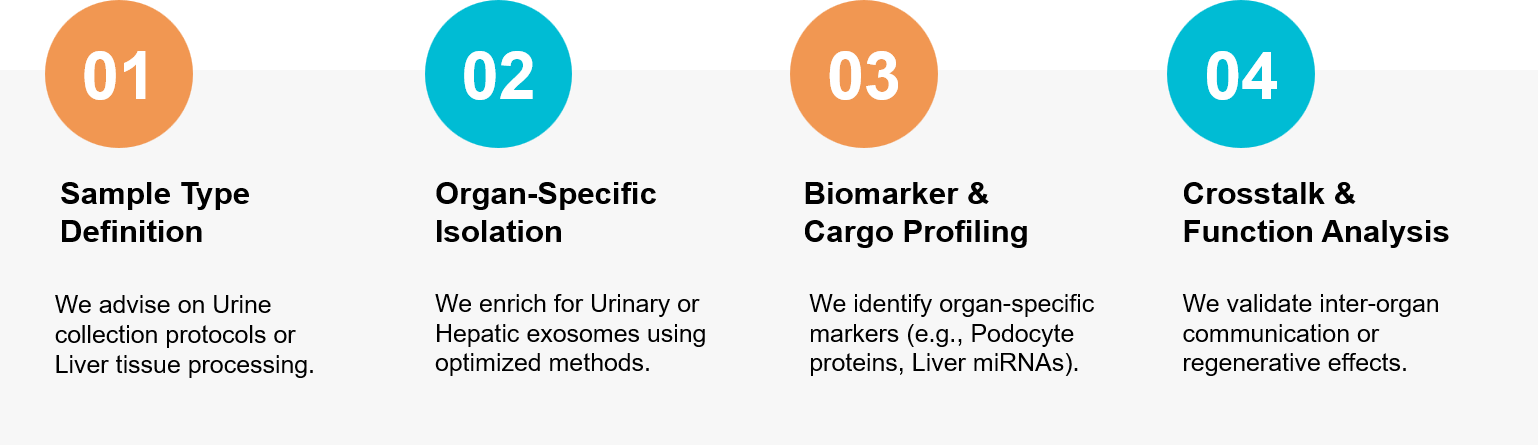 Figure 3. Our integrated workflow for discovering urinary biomarkers and mapping liver-organ communication. (Creative Biostructure)
Figure 3. Our integrated workflow for discovering urinary biomarkers and mapping liver-organ communication. (Creative Biostructure)
Ready to explore the potential of liquid biopsy and organ crosstalk? Our scientific team is available for a free consultation to discuss your renal or hepatic exosome strategy.
References
- Oshikawa S, Sonoda H, Ikeda M. Aquaporins in Urinary Extracellular Vesicles (Exosomes). Int J Mol Sci. 2016 Jun 17;17(6):957.
- Costa-Silva B, Aiello NM, Ocean AJ, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017 Feb 23;542(7642):450-455.