Exosome Immunomodulation and Inflammation Control Studies
Chronic inflammation and autoimmune diseases are driven by an imbalance in the immune system. While corticosteroids and monoclonal antibodies effectively suppress symptoms, they often lead to broad immunosuppression and severe side effects. Mesenchymal Stem Cell (MSC) Exosomes offer a paradigm shift: they do not just suppress; they regulate.
We provide comprehensive Exosome Immunomodulation Solutions. We help you harness the natural ability of exosomes to reprogram immune cells—shifting macrophages from pro-inflammatory (M1) to restorative (M2) phenotypes and boosting Regulatory T cells (Tregs). Our platform enables the development of cell-free therapeutics for conditions ranging from cytokine release syndrome to autoimmune disorders, ensuring potency without the risks of live cell therapy.
Mechanisms of Exosomal Immunoregulation
Why are exosomes superior regulators of inflammation? They carry a complex cocktail of bioactive molecules that act on multiple immune pathways simultaneously.
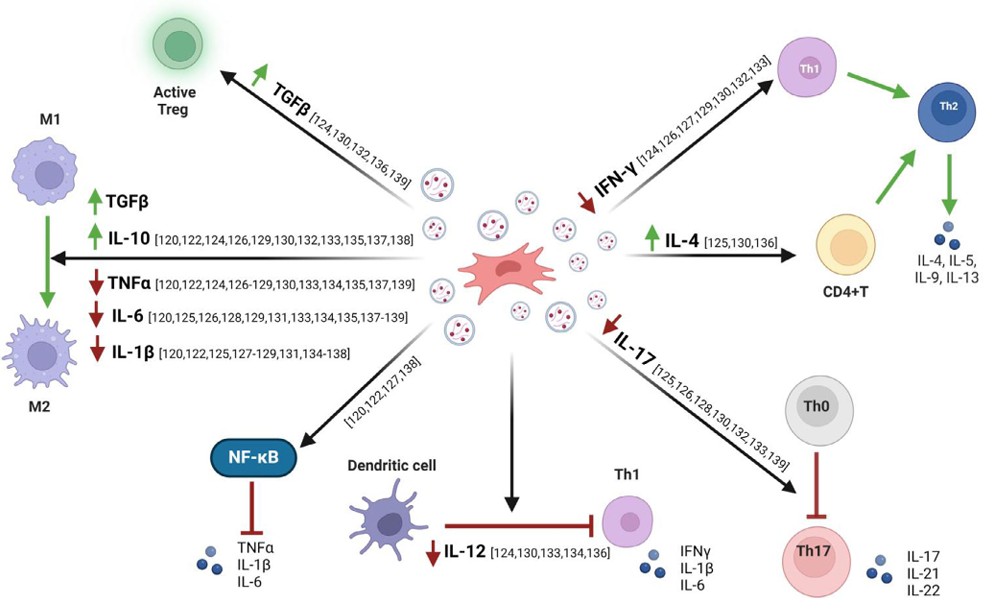
- Macrophage Polarization: MSC exosomes carry specific miRNAs (e.g., miR-146a, miR-181c) that actively switch macrophages from an inflammatory "attack" mode (M1) to a "repair" mode (M2), resolving tissue inflammation at the source.
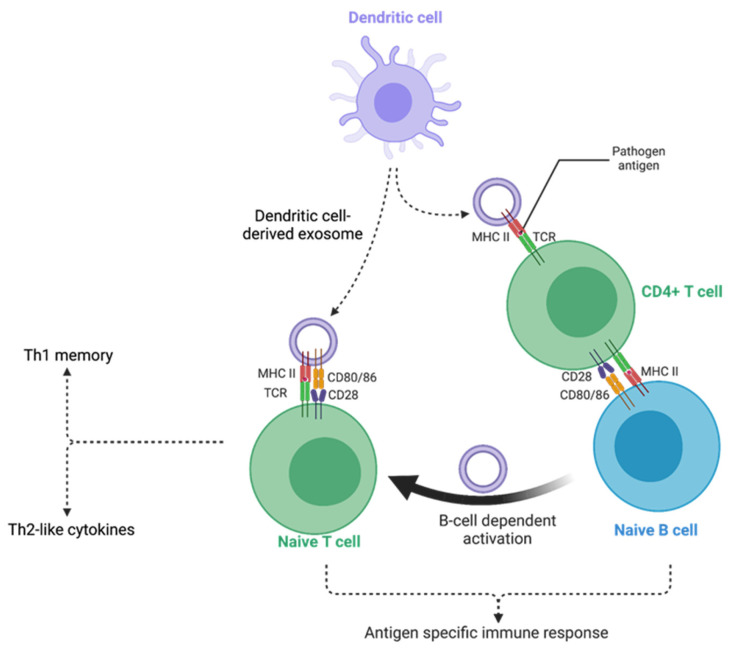
- T-Cell Regulation: Exosomes can inhibit the proliferation of effector T cells and promote the expansion of Regulatory T cells (Tregs), which are crucial for maintaining self-tolerance in autoimmune diseases.
- Cytokine Suppression: By inhibiting the NF-κB signaling pathway, exosomes significantly reduce the secretion of "Cytokine Storm" drivers like TNF-α, IL-1β, and IL-6.
- Safety Profile: Unlike live stem cells, exosomes do not express MHC class II molecules at high levels, minimizing the risk of allogeneic rejection while maintaining robust immunomodulatory potency.
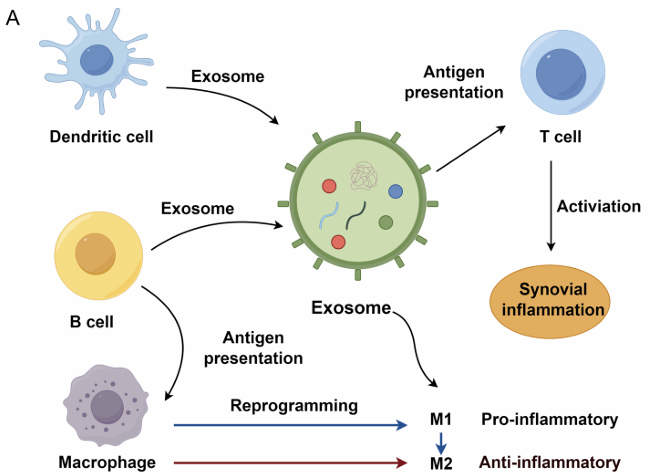 Figure 1. Exosomes from immune cells facilitate synovial inflammation in osteoarthritis. (Lan S, et al., 2025)
Figure 1. Exosomes from immune cells facilitate synovial inflammation in osteoarthritis. (Lan S, et al., 2025)
Our Immunomodulation Development Workflow
We offer a specialized pipeline to isolate potent exosomes and validate their immune-dampening effects.
| Development Phase | Our Specialized Approach & Solution | Key Services Applied |
|---|---|---|
| Source Optimization | Priming Strategy: Exosomes from resting MSCs may have low potency. We use inflammatory priming (e.g., treating MSCs with IFN-γ or TNF-α) during culture to stimulate the production of "super-charged" anti-inflammatory exosomes. | Upstream Process Development (Cell Culture Optimization) |
| Cargo Profiling | Mechanism of Action: We profile the exosomal cargo to identify the specific anti-inflammatory mediators. This includes miRNA Sequencing to find regulators of immune pathways and Proteomics to detect cytokines like TGF-β and IL-10. | Exosomal Small RNA and miRNA Sequencing, Exosome Proteomics |
| In Vitro Assays | Immune Cell Models: We utilize co-culture systems with activated PBMCs, macrophages, or Dendritic Cells. We measure endpoints like T-cell proliferation inhibition (CFSE assay) and macrophage phenotype switching (Flow Cytometry). | Immunomodulation and Inflammation Assays |
| In Vivo Validation | Disease Models: We validate efficacy in relevant animal models, such as Dextran Sulfate Sodium (DSS)-induced colitis (for IBD) or Collagen-Induced Arthritis (for RA), quantifying inflammation reduction and tissue repair. | In Vivo Exosome Functional Assays |
Core Technologies for Immune Therapies
We deploy specific assays to quantify the abstract concept of "immunomodulation."
Macrophage Polarization Assays
Visualizing the Switch: The shift from M1 to M2 is the gold standard for anti-inflammatory potency. We use Flow Cytometry to quantify surface markers (CD86 for M1 vs. CD206 for M2) and ELISA to measure the ratio of secreted cytokines (IL-10/IL-12). This assay provides definitive proof that your exosomes are actively resolving inflammation.
Inflammatory Priming (Preconditioning)
Enhancing Potency: Standard exosomes might not be enough for severe diseases. We offer Cell Culture Optimization services where we "license" the producer cells with inflammatory cytokines (IFN-γ). This mimics the disease environment, triggering the MSCs to package higher levels of anti-inflammatory proteins (like IDO, COX-2) into the exosomes, significantly boosting their therapeutic index.
Cytokine Array Profiling
Broad-Spectrum Analysis: Inflammation is complex. Instead of measuring one marker, we use Multiplex Cytokine Arrays to simultaneously screen up to 40+ inflammatory mediators. This allows us to map the global effect of your exosome treatment on the inflammatory network, identifying both downregulated pro-inflammatory markers and upregulated anti-inflammatory factors.
Application Spotlight: MSC Exosomes for Resolving Lung Inflammation
This analysis highlights how "priming" MSCs can create superior exosomes for treating severe inflammatory conditions like Acute Lung Injury (ALI).
Featured Technologies:
- Inflammatory Priming (Cell Culture)
- Macrophage Functional Assays
Literature Interpretation:
Treating acute inflammation requires a potent therapeutic intervention. Researchers investigated whether pre-treating (priming) MSCs with Lipopolysaccharide (LPS) could enhance the therapeutic efficacy of their exosomes compared to naive exosomes. They found that LPS-primed MSC exosomes were significantly more effective at converting macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype. This enhanced therapeutic effect was primarily driven by the enrichment of a specific microRNA, let-7b, in the primed exosomes, which actively regulated the TLR4/NF-κB pathway to resolve chronic inflammation and promote tissue repair.
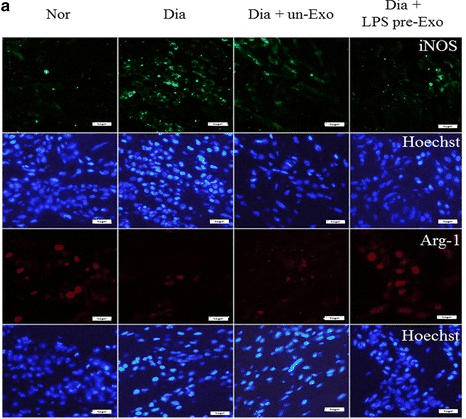 Figure 2. Macrophage phenotype distribution in wound sites of diabetic rats treated with LPS pre-Exo for 3 days, showing immunofluorescence staining for iNOS (M1, green), Arg1 (M2, red), and nucleus (blue). (Ti D, et al., 2015)
Figure 2. Macrophage phenotype distribution in wound sites of diabetic rats treated with LPS pre-Exo for 3 days, showing immunofluorescence staining for iNOS (M1, green), Arg1 (M2, red), and nucleus (blue). (Ti D, et al., 2015)
Start Your Immune Therapy Project
We make getting started straightforward. Our process is designed to be collaborative and transparent.
How It Works: Our Project Pathway
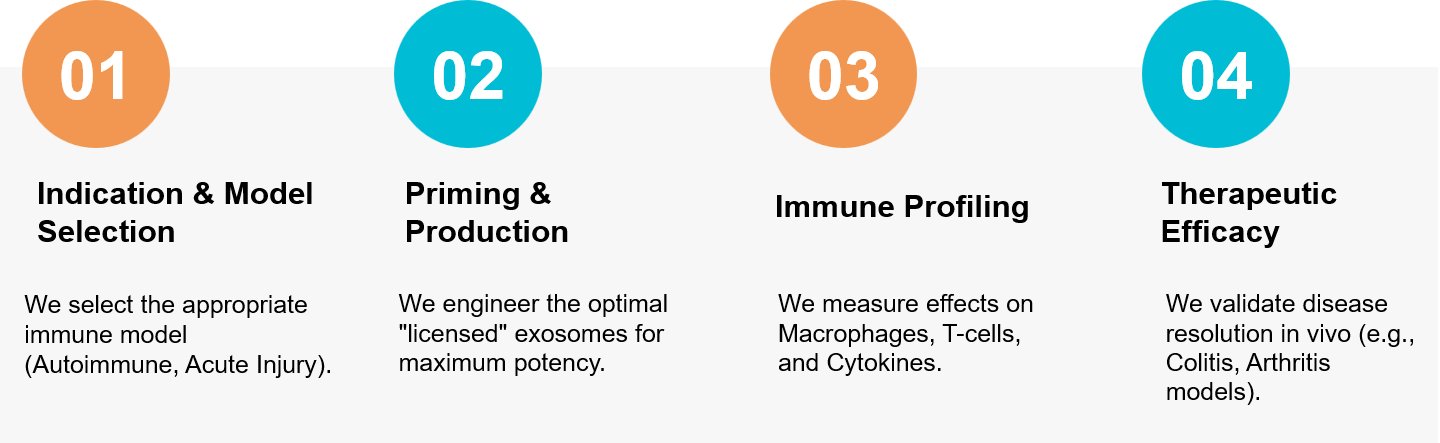 Figure 3. Our workflow for engineering "primed" exosomes to regulate immune responses and resolve chronic inflammation. (Creative Biostructure)
Figure 3. Our workflow for engineering "primed" exosomes to regulate immune responses and resolve chronic inflammation. (Creative Biostructure)
Ready to develop a safer alternative to immunosuppressants? Our scientific team is available for a free consultation to discuss your immunomodulation strategy. Contact us today to discuss your project.
References
- Lan S, Zhang C. Roles of exosomes in immune regulation of osteoarthritis and their applications in inflammation repair. Front Immunol. 2025 Sep 4;16:1611718.
- Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015 Sep 19;13:308.