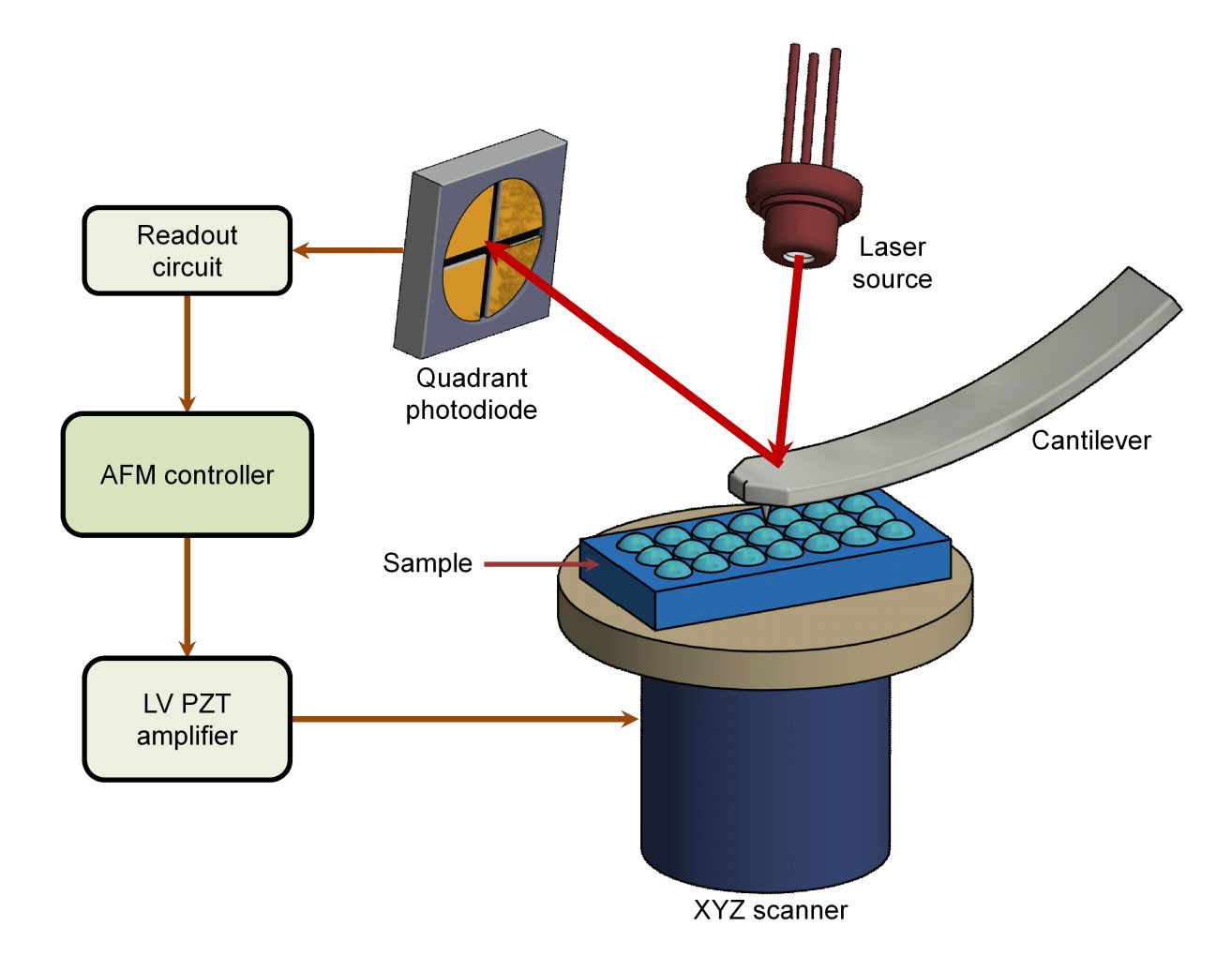
Transmission Electron Microscopy (TEM)-Based Exosome Characterization Service
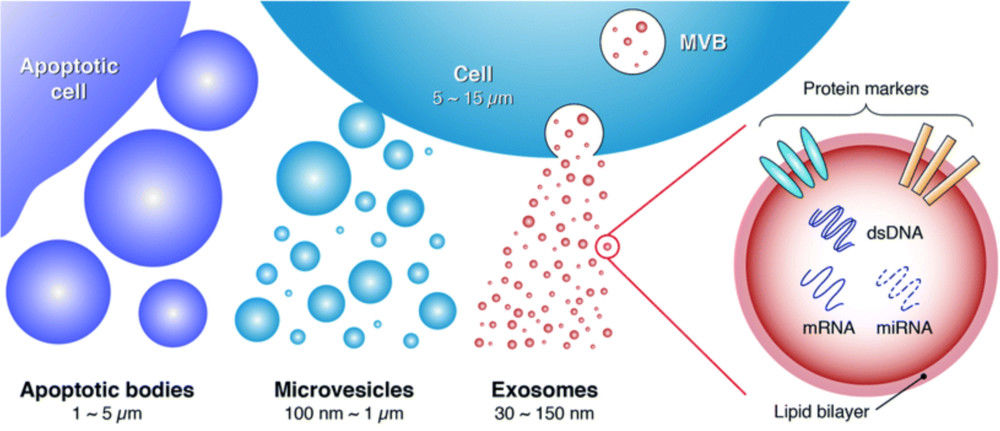
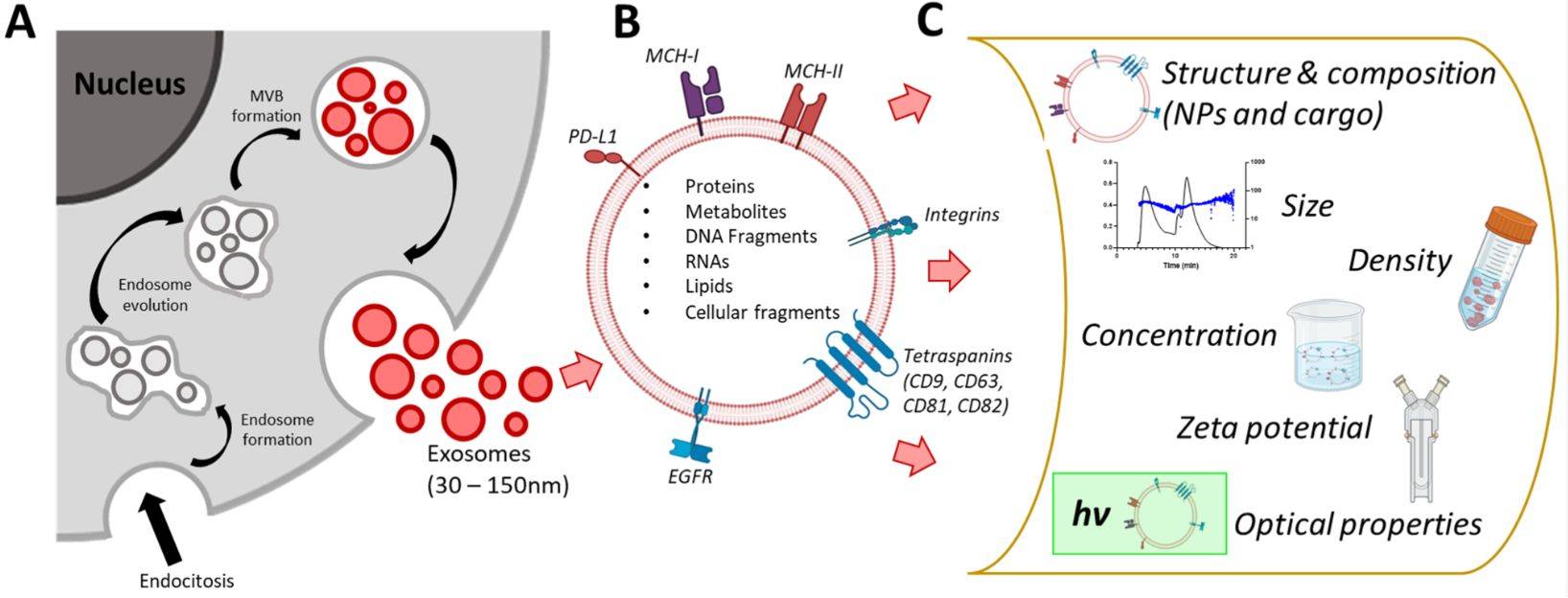
Exosomes are nanoscale extracellular vesicles (EVs) that play vital roles in intercellular communication, disease progression, and therapeutic delivery. Accurate characterization of their structure is essential for both basic research and translational applications. Among available imaging techniques, Transmission Electron Microscopy (TEM) is regarded as the gold standard for confirming the morphology, size, and structural integrity of exosomes.
At Creative Biostructure, we provide comprehensive TEM-based exosome characterization services following international standards such as the MISEV2023 guidelines, ensuring reliable, reproducible, and publication-ready results for academic and industrial clients.
What is TEM Analysis of Exosomes?
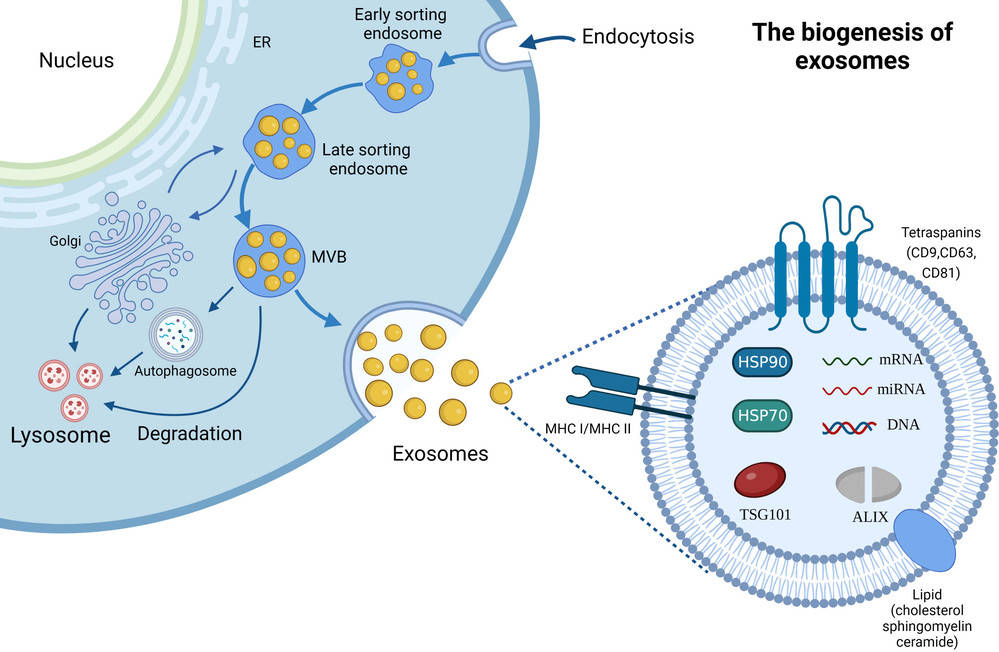
TEM analysis of exosomes involves directing a high-energy electron beam through exosome samples mounted on grids. The resulting interaction produces high-resolution images that reveal:
- Morphology: vesicular cup-shaped or spherical appearance.
- Size range: typically 30-150 nm.
- Membrane structure: visualization of the lipid bilayer.
- Integrity and purity: distinguishing intact vesicles from aggregates, protein contaminants, or debris.
This level of detail allows researchers to validate exosome isolation, assess purity, and confirm suitability for downstream applications such as biomarker discovery, therapeutic engineering, or clinical translation.
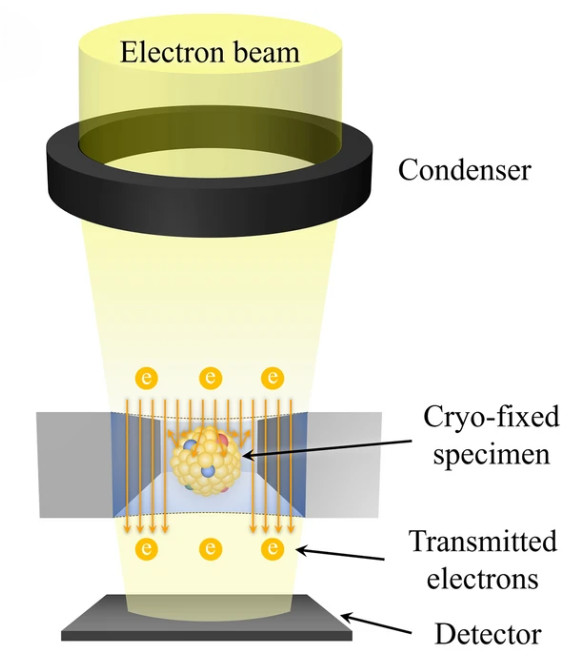
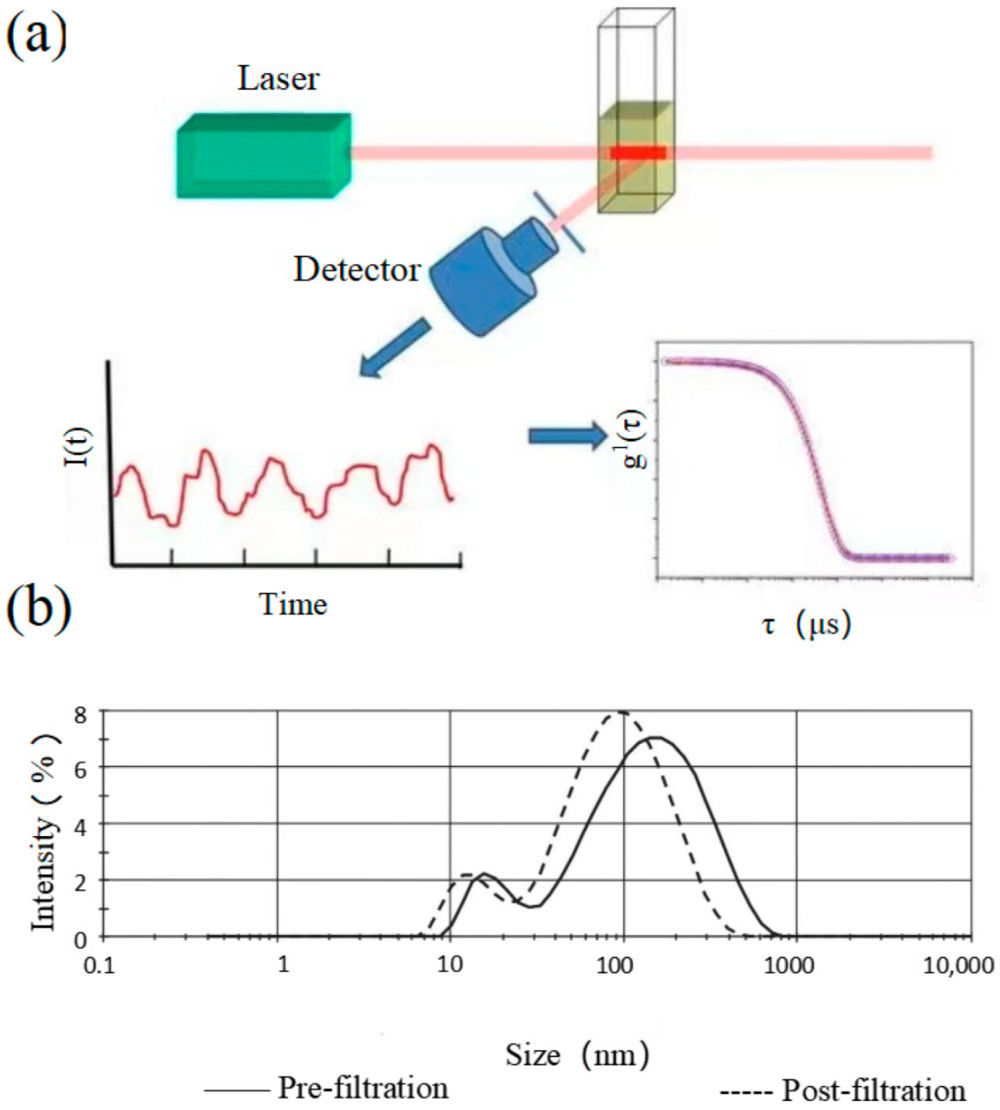
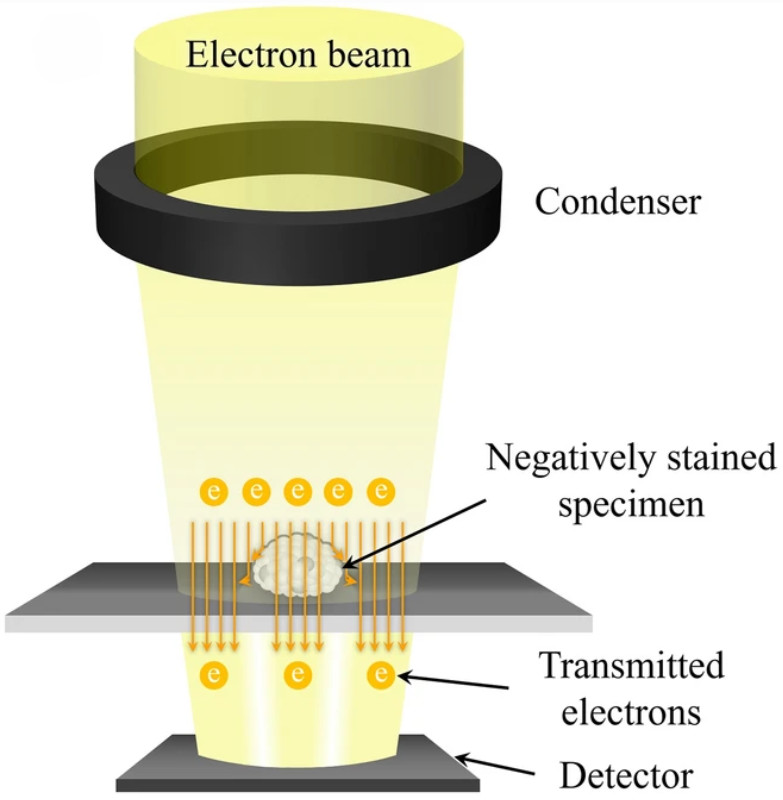 Figure 1. TEM captures images of extracellular vesicles by detecting electrons transmitted through negatively stained samples. (Kwon Y, et al., 2022)
Figure 1. TEM captures images of extracellular vesicles by detecting electrons transmitted through negatively stained samples. (Kwon Y, et al., 2022)
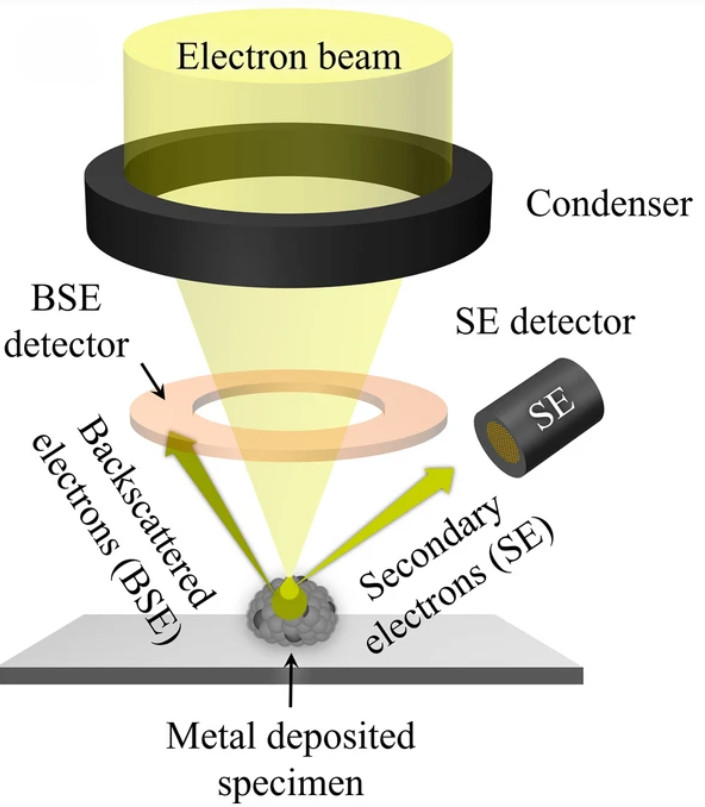
TEM vs. Cryo-TEM and SEM for Exosome Characterization
| Feature | TEM | Cryo-TEM | SEM |
|---|---|---|---|
| Principle | Electron beam passes through fixed and stained samples | Samples rapidly vitrified at cryogenic temperatures to preserve native state | Electron beam scans the surface of vesicles |
| Sample Prep | Fixation, adsorption, negative staining | Vitrification without chemical fixation | Surface coating required |
| Resolution | High resolution, morphology and bilayer visible | Near-native state, lipid bilayer clearly visible | Excellent for surface topography |
| Sample Integrity | Dehydration may slightly alter morphology | Preserves natural morphology and hydration | Limited for internal structure |
| Best Use | Routine exosome morphology and size validation | Functional, structural, and near-native analysis | Surface characterization |
Our TEM Exosome Characterization Workflow
At Creative Biostructure, we follow a standardized yet flexible workflow that ensures scientific rigor, reproducibility, and efficient project delivery. From the first consultation to the final report, every step is carefully managed by our expert team.
Initial Consultation
Our scientific team works closely with clients to clarify project goals and sample types while providing detailed guidance on preparation, concentration, and storage to ensure sample integrity prior to shipment.
Sample Submission and Quality Check
Clients may submit either purified exosomes or original biological materials; we perform exosome isolation if needed, followed by quality checks on concentration and purity to ensure suitability for TEM analysis in compliance with MISEV2023 guidelines.
Sample Preparation for TEM
• Fixation: controlled fixation with appropriate reagents to maintain vesicle structure.
• Adsorption: deposition of vesicles onto carbon-coated TEM grids.
• Negative Staining: application of uranyl acetate and methylcellulose to enhance contrast and preserve bilayer morphology.
Grid Processing and Imaging
We carefully handle TEM grids to avoid artifacts or aggregation and perform multi-magnification imaging:
• Low magnification for assessing vesicle distribution.
• High magnification for resolving individual exosome morphology and bilayer details.
Data Acquisition and Image Analysis
We capture high-resolution images across multiple fields of view, conduct statistical analyses of exosome size, morphology, and integrity, and optionally compare with engineered or disease-associated vesicles.
Comprehensive Reporting
We provide raw and publication-ready TEM images along with a detailed analytical report covering methodology, representative images, size distribution, and expert interpretation, formatted to meet publication, grant, and regulatory requirements.
Follow-Up Support
Post-analysis consultation to discuss results, answer technical questions, and provide recommendations for next steps (e.g., complementary assays such as nanoparticle tracking analysis, flow cytometry, or proteomics). Long-term collaboration available for clients requiring ongoing exosome characterization or therapeutic development support.
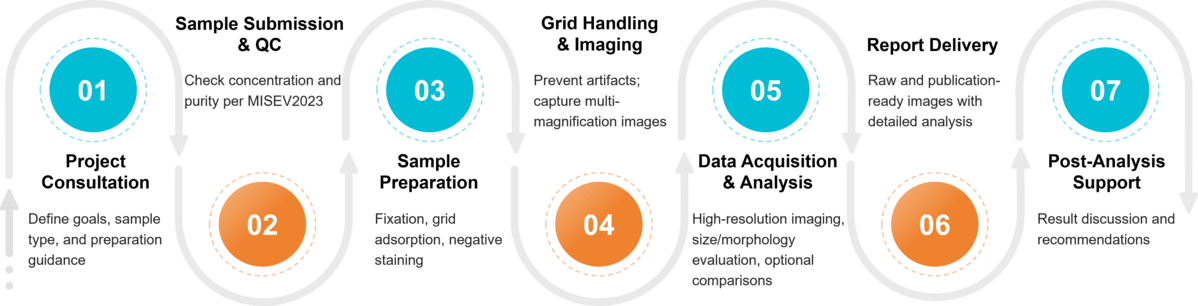 Figure 2. Project Workflow for Exosome Characterization by Transmission Electron Microscopy. (Creative Biostructure)
Figure 2. Project Workflow for Exosome Characterization by Transmission Electron Microscopy. (Creative Biostructure)
Scientific Standards and Guidelines
We strictly adhere to MISEV2023 recommendations for electron microscopy characterization of extracellular vesicles:
- Fixation: clear reporting of fixative type, concentration, and incubation time.
- Adsorption: grid material, mesh size, coating, incubation, and wash details.
- Negative staining: reagent type, concentration, and incubation time.
- Image documentation: both low- and high-magnification images to assess overall sample quality and fine vesicular details.
Sample Requirements for TEM Analysis
To ensure reliable and high-quality TEM results, we recommend the following sample requirements:
| Requirement | Details | Notes |
|---|---|---|
| Sample Type | Purified exosomes or original biological materials (cell culture supernatant, plasma, serum, urine, saliva, milk, plant extracts) | If original material is provided, Creative Biostructure can perform exosome isolation and purification prior to TEM imaging |
| Volume | ≥ 20 µL for purified exosome samples | Larger volumes may be required for original biological fluids depending on concentration |
| Concentration | ≥ 1 × 1010 particles/mL (recommended) | Lower concentrations may reduce image quality and statistical reliability |
| Buffer | Sterile PBS (pH 7.2-7.4) | Avoid buffers containing high concentrations of proteins or detergents |
| Storage Conditions | Store at 4 °C for short-term (<48 h); -80 °C for long-term | Avoid repeated freeze-thaw cycles to prevent vesicle rupture or aggregation |
| Additional Information | Provide details of isolation method, storage history, and any prior treatments | Ensures accurate interpretation and compliance with MISEV2023 reporting standards |
What Deliverables Will You Receive
- Raw TEM images of exosome samples at multiple magnifications
- Publication-ready processed images with optimized contrast and clarity
- Comprehensive analytical report detailing methodology, representative images, and size distribution data
- Expert interpretation of morphology, integrity, and purity
- Optional comparative analysis for engineered or disease-associated exosomes
Applications of TEM-Based Exosome Characterization
- Morphological confirmation of exosome samples.
- Purity and quality assessment, including detection of aggregates, cell debris, or protein contaminants.
- Structural validation of engineered exosomes, such as drug- or RNA-loaded vesicles.
- Evaluation of exosomes in disease research, including oncology, neurodegeneration, and metabolic disorders.
- Quality control for therapeutic development, ensuring vesicle stability and integrity before clinical use.
- Plant- or fluid-derived exosome analysis, extending applications to agriculture, food science, and biomarker discovery.
Advantages of Our TEM Exosome Service
- Advanced Instrumentation: state-of-the-art TEM systems with nanometer-scale resolution.
- MISEV-Compliant Protocols: standardized sample preparation and reporting for reproducibility.
- Experienced Scientists: extensive expertise in exosome isolation, EM imaging, and data interpretation.
- Customizable Solutions: tailored protocols for engineered exosomes, cryo-TEM, or SEM integration.
- High-Quality Deliverables: publication-ready TEM images and professional analysis reports.
Case Study
Case: TEM Characterization of hucMSC-Derived Exosomes in Cardioprotection
Background
Acute myocardial infarction and ischemia/reperfusion injury often cause cardiomyocyte death through inflammasome activation and pyroptosis. Exosomes from human umbilical cord mesenchymal stem cells (hucMSCs) are rich in bioactive miRNAs and may provide protective effects. This study explored whether hucMSC-derived exosomes could reduce hypoxia/reoxygenation (H/R)-induced pyroptosis via the miR-100-5p/FOXO3/NLRP3 pathway.
Methods
- Isolation: hucMSC-derived exosomes were isolated and purified.
- Characterization: Creative Biostructure performed TEM analysis, confirming typical morphology and intact structure, with CD9, CD63, and Alix validated by Western blot.
- Functional Assays: Exosome-treated AC16 cardiomyocytes were tested for cell viability, LDH release, inflammasome activity, and cytokine secretion.
- Mechanistic Studies: miR-100-5p expression was modulated to evaluate its effect on FOXO3/NLRP3 signaling.
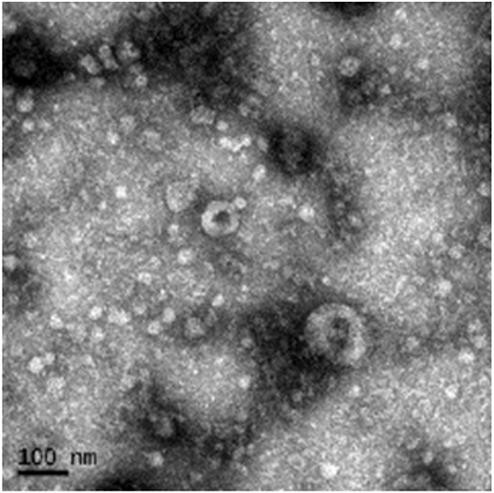 Figure 3. TEM Image of Purified hucMSC-Derived Exosomes. (Liang C, et al., 2021)
Figure 3. TEM Image of Purified hucMSC-Derived Exosomes. (Liang C, et al., 2021)
Results
- TEM Imaging (Our Contribution): TEM confirmed vesicle integrity and purity, providing key morphological evidence.
- Exosomes improved cell viability, reduced pyroptosis, and suppressed NLRP3, caspase-1, GSDMD, IL-1β, and IL-18 under H/R stress.
- miR-100-5p directly targeted FOXO3, downregulating NLRP3 activation. FOXO3 overexpression reversed these protective effects.
Conclusion
hucMSC-derived exosomes protected cardiomyocytes from H/R-induced pyroptosis through the miR-100-5p/FOXO3/NLRP3 pathway. Creative Biostructure's TEM characterization confirmed exosome morphology and quality, supporting reliable mechanistic insights and strengthening the study's findings.
At Creative Biostructure, we combine advanced instrumentation, standardized protocols, and expert interpretation to deliver reliable TEM exosome characterization results. Whether you require morphological confirmation, quality assessment, or validation of engineered vesicles, our team is ready to support your research and development. Contact us today to discuss your project.
References
- Liang C, Liu Y, Xu H, et al. Exosomes of human umbilical cord MSCs protect against hypoxia/reoxygenation-induced pyroptosis of cardiomyocytes via the miRNA-100-5p/FOXO3/NLRP3 pathway. Frontiers in Bioengineering and Biotechnology. 2021, 8: 615850.
- Kwon Y, Park J. Methods to analyze extracellular vesicles at single particle level. Micro and Nano Systems Letters. 2022, 10(1): 14.
- Welsh J A, Goberdhan D C I, O'Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles. 2024, 13(2): e12404.
Frequently Asked Questions
For any inquiries, our support team is ready to help you get technical support for your research and maximize your experience with Creative Biostructure.