Custom MemPro™ LRR-Containing Membrane Proteins Services
Creative Biostructure provides custom MemPro™ gene-to-structure services for Leucine rich repeat (LRR)-containing membrane proteins. Specialists from Creative Biostructure serve our customers with tools and approaches for the production, characterization and crystallization of integral membrane proteins.
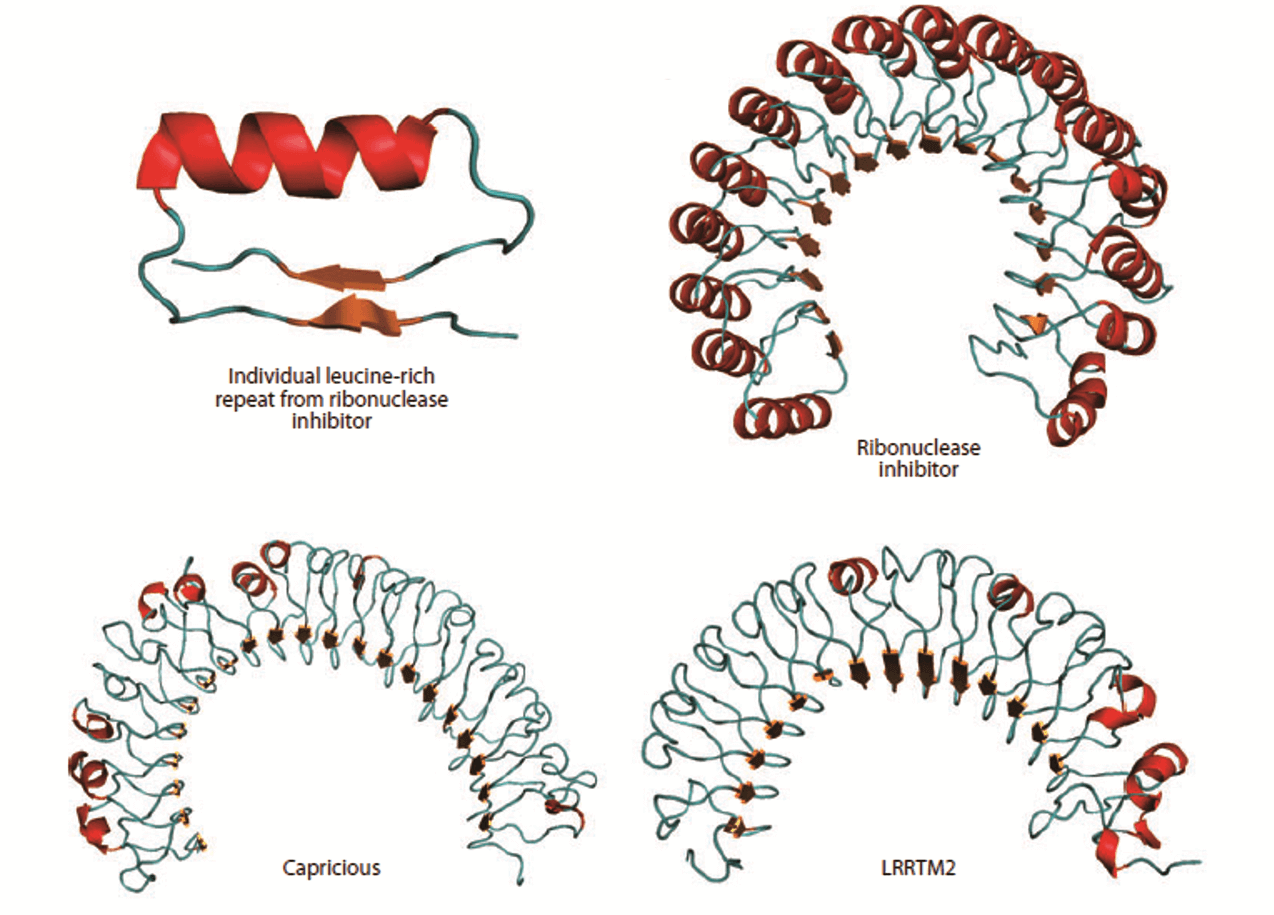 Figure 1. Structure of the leucine-rich repeat (LRR) domain. (Annu. Rev. Cell Dev. Biol., 2011)
Figure 1. Structure of the leucine-rich repeat (LRR) domain. (Annu. Rev. Cell Dev. Biol., 2011)
One of the typical protein repeat domains, the leucine-rich repeat (LRR), is a structural motif of 20-30 amino acid stretches that are abundant in the hydrophobic leucine residue. In general, most transmembrane proteins contain the LRR. The N-terminus of LRR is composed of a conserved 11-residue sequence rich in leucine. It has been reported that this part of the motif forms a β-strand and a loop region. The C-terminus of LRR that interacts with the loop region of the N-terminal part. is more variable in sequence and structure. The strand-turn-helix structure of LRR repeat unit is the assembled domain that has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. Usually, this domain is a tandem array of a few (or more) individual LRR motifs. LRR-containing transmembrane proteins, which commonly function as synapse organizers, are important for the regulation of protein-protein interactions found in innate immunity and nervous system development. For instance, six LRR proteins are located in postsynapses, most of which are capable of inducing presynaptic differentiation in artificial synapse formation assays. Four genes of LRR-containing transmembrane proteins are found only in vertebrates and possess similar domain structures comprising 10 LRRs, a single transmembrane segment and a short cytoplasmic stretch. LRR motifs are identical in the extracellular domains of membrane proteins and in secreted proteins associated with ligand-receptor binding or cell adhesion. The structures and lengths of LRR domains determine the various architectures for different protein-ligand interactions. Thus, proteins containing extracellular LRR domains are well suitable for regulating intercellular communication and cell-to-cell adhesion. Creative Biostructure offers you production, computational modeling and structural determination of LRR-containing transmembrane proteins, depending on the needs of customers.
Creative Biostructure can provide various MemPro™ gene-to-structure services for membrane protein family, including the identification, isolation, purification, stabilization, and crystallization of membrane proteins of your interest. Please feel free to contact us for a detailed quote.
References:
J. de Wit et al. (2011). Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu. Rev. Cell Dev. Biol., 27: 697-729.
J. Ko (2012). The Leucine-Rich Repeat superfamily of synaptic adhesion molecules: LRRTMs and slitrks. Mol. Cells, 34: 335-340.
Leucine-rich repeat. (https://en.wikipedia.org/wiki/Leucine-rich_repeat)