Mempro™ Virus-Like Particles (VLPs) Protein Attachment Modification
Creative Biostructure has developed various methods for appropriate modification of virus-like particles (VLPs). Based on the advanced Mempro™ VLPs platform established for years, scientists from Creative Biostructure can perform unique Mempro™ VLPs protein modification services.
VLPs are composed of structural proteins of viruses and they are non-infectious protein structures which can self-assemble into either icosahedral or rod-like structures. When using chimeric VLPs as commercial vaccines they have several limitation, for example, self-assembly of chimeras is extremely unpredictable, the length of the fused antigen is representatively restricted and non-peptide antigens cannot be incorporated. By contrast, Creative Biostructure provides modular approaches which can offer a more strong and flexible means for displaying antigens on the surface of VLPs.
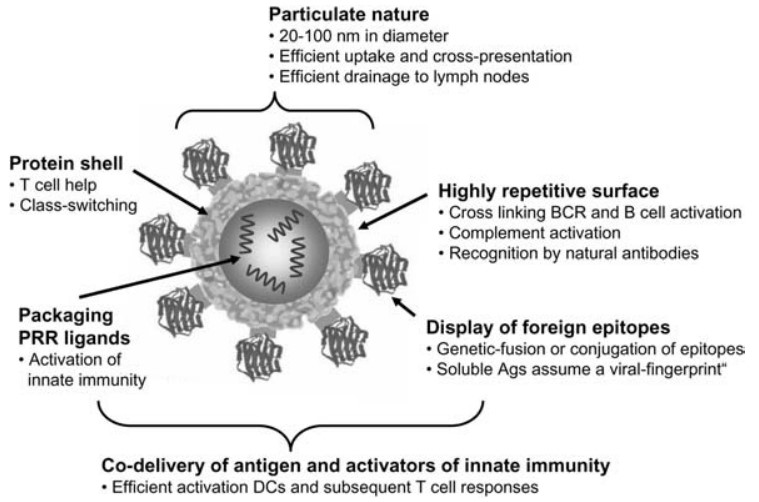 Figure 1. Immunogenic features of a VLP presenting foreign antigens. (Biol. Chem 2008)
Figure 1. Immunogenic features of a VLP presenting foreign antigens. (Biol. Chem 2008)
- Approaches of Virus-Like Particles (VLPs) Protein Attachment Modification
To display antigens on the surface of VLPs, using covalent linkage is the most suitably and flexible way. This enable to be accomplished by the use of chemical cross-linkers, moreover, various conjugation strategies exist to reach this end. For example, use hetero-bifunctional conjugation reagents having two evident reactive groups which can bond to different and distinct functional targets, one on the antigen and the other on the VLP (typically amines or sulfhydryl residues). Another approach to present antigens is non-covalent attachment of proteins to the surface of VLPs. For example, researches have developed a method based on the strong interaction between streptavidin and biotin, which has triumphantly bonded recombinantly produced streptavidin-tumor necrosis factor a (TNFa) peptide fusions to biotinylated HPV VLPs and used to produce neutralizing anti-TNFa antibodies.
- Advantages of Virus-Like Particles (VLPs) Protein Attachment Modification
The antigens and VLPs are following assembled in vitro through either covalent or non-covalent binding to link the antigen to the surface of the preassembled VLP. One of the advantages of this methods is that the structure and size of the recombinant target antigen are not restricted by the folding conditions of the VLP monomer and by particle assembly. The opportunity of combining a fulllength, exactly folded target protein with the VLP can be obviously increased by independent synthesis of the recombinant antigen, therefore maximizes vaccine quality.
Creative Biostructure has been pioneered in the VLPs production for a long time, we can provide various Mempro™ VLPs modification strategies services. Please feel free to contact us for a detailed quote.
References:
Jennings G T, Bachmann M F. The coming of age of virus-like particle vaccines[J]. Biological chemistry, 2008, 389(5): 521-536.
Chackerian B. Virus-like particles: flexible platforms for vaccine development[J]. Expert review of vaccines, 2007, 6(3): 381-390.
Chackerian B, Lowy D R, Schiller J T. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies[J]. The Journal of clinical investigation, 2001, 108(3): 415-423.
Hooker J M, Kovacs E W, Francis M B. Interior surface modification of bacteriophage MS2[J]. Journal of the American Chemical Society, 2004, 126(12): 3718-3719.