Custom MemPro™ Peptidase AD Services
Peptidase AD, alternative name gamma secretase complex plays a pivotal role in the progress of Alzheimer’s disease (AD). They generate the cleavage of amyloid precursor protein (APP) which is the parent molecule of beta amyloid protein (Aβ). Peptidase AD is an 18-times transmembrane aspartyl protease to regulate intramembrane proteolysis for a growing list of integral membrane proteins.
It contains four core components: presenilins, nicastrin, anterior pharynx defective (aph-1) and presenilin enhancer 2 (pen-2).
- Presenilins, are nine-pass transmembrane proteins and considered to possess enzyme catalytic activity.
- Nicastrin is a single pass membrane protein with a large ectodomain that is heavily glycosylated which plays help gamma-secretase assembling.
- Aph-1 occurs as a seven-pass transmembrane protein that could be further divided into two homologous forms, which located on chromosome 1 and chromosome 15 (aph-1a & aph-1b) respectively; Aph-1a undergoes further splicing to generate a long and short isoform of aph-1a, with the short isoform more abundantly expressed in most tissues. Aph-1 shares its function with nicastrin in forming the stable complex.
- The smallest component Pen-2 is a two-pass transmembrane protein that is thought to activate presenilin endoproteolysis. These proteins are essential for gamma-secretase activity.
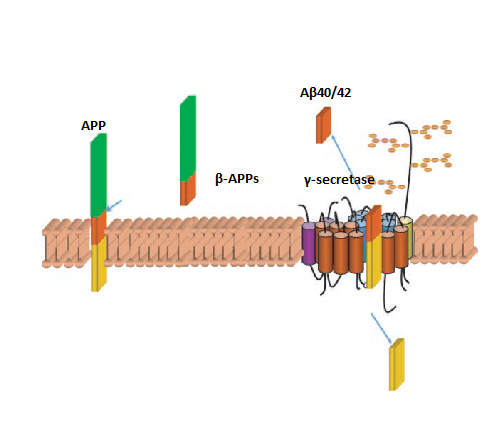 Figure 1. Amyloidogenic (Aβ generating) pathway
Figure 1. Amyloidogenic (Aβ generating) pathway
Thus, overall the enzyme possesses 18 transmembrane domains making it difficult to elucidate the crystal structure of the enzyme. The proteins are adapted to the lipid bi-layer and are easily to denature and lost biological functions when extracted from the environment. The region of hydrophobic residues will require specific procedure to be stabilized by detergents during preparation for structural analysis. High resolution X- ray crystallography methods have improved in which 3D crystallization methods can be used.
Several drugs act as gamma-secretase inhibitors have been developed to treat Alzheimer’s diseases. However, since gamma-secretase have more than 50 different substrate, concerns have been about the specificity, selectivity and toxicity of agents aimed at inhibiting or modulating gamma-secretase activity. Meanwhile, the reason about the process of Alzheimer’s diseases still remains unclear and gamma-secretase inhibitors can only treat symptoms without targeting the underlying pathology or neurodegeneration.
References:
Krishnaswamy S, Verdile G, Groth D, et al. The structure and function of Alzheimer’s gamma secretase enzyme complex[J]. Critical reviews in clinical laboratory sciences, 2009, 46(5-6): 282-301.