Custom MemPro™ Soluble N-ethylmaleimide Sensitive Factor Attachment Protein Receptor (SNARE) Complex
Creative Biostructure has developed custom MemPro™ gene-to-structure services for the SNARE complex. The superfamily of SNARE complex contains 3 families: SNARE complex, synaptobrevin and syntaxin.
SNARE complex can mediate vesicle fusion between two membranes. In neuronal SNAREs, synaptobrevin, syntaxin and SNAP-25 contribute to the SNARE motifs (60-70 amino acids): one from each of synaptobrevin and syntaxin while two from SNAP-25 to form a four-helix bundle. In forming the zero ionic layer, synaptobrevin located in the synaptic vesicle contributes a glutamine (Q).while syntaxinor SNAP-25 contribute an arginine (R).Based on this, SNAREs can be divided into two types, R-SNAREs and Q-SNAREs. Q-SNAREs can be further classified into three subgroups, Qa, Qb and Qc.
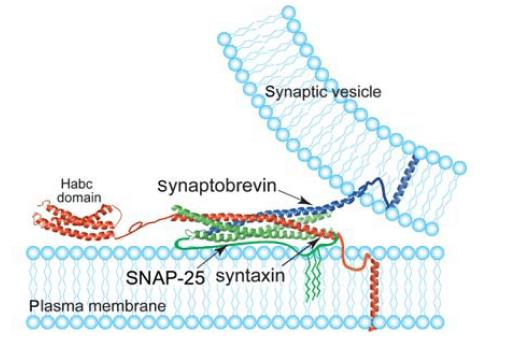 Figure. The core SNARE complex
Figure. The core SNARE complex
In eukaryotic cells, membrane fusion is very important for various physiological processes, such as hormone secretion, protein and membrane trafficking, neurotransmission, etc. SNARE proteins, ubiquitous proteins, play a crucial role in these processes. SNARE proteins consist of a “SNARE” domain flanked by a C-terminal single-helical transmembrane anchor and a variable N-terminal domain. The transmembrane segments (TMSs) contribute to SNARE anchoring to membranes and SNARE-SNARE interaction. While the SNAREs lacking TMS, such as Q-SNAREs SNAP-25 and SNAP-23, are anchored to membranes by palmitate anchors. As shown in the figure, SNARE proteins zipper into a stable four-helix bundle to drive membrane fusion. The source of vesicles, the identity of target membranes and other factors including complexins contribute to the combinations of SNARE proteins and the specificity of the membrane targeting. Using the energy from ATP hydrolysis, the ATPase NSF and SNAPs (soluble NSF attachment protein) can disassemble non-productive and post-fusion SNARE complexes into individual components to maintain the pool of individual SNARE proteins. NSF is a member of AAA-ATPase, contributing to eukaryotic trafficking. It forms a homomeric hexamer. NSF, together with SNAP and SNARE complex, forms 20S supercomplex before ATP hydrolyzes. And prior to fusion and after fusion, NSF and α/γ-SNAPs facilitate SNARE complexes to dissociate to reuse.
Creative Biostructure can provide custom MemPro™ gene-to-structure services for membrane proteins. Please click for more information.
Mark T. Palfreyman and Erik M. Jorgensen. Roles of SNARE Proteins in Synaptic Vesicle Fusion. Molecular Mechanisms of Neurotransmitter Release. 35-59.
Minglei Zhao, et al. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015 Feb 5;518(7537):61-7.