MemPro™ EPHA2
Creative Biostructure provides custom MemPro™ gene-to-structure services for EPHA2.
Erythropoietin-producing hepatocellular receptor family (EPH receptor), is one of the largest group among the tyrosine kinase receptor families. It comprises of the EphA (EphA1 -- 10) or EphB (EphB1 -- 6) subclasses of receptors classified as per their sequence homologies and their binding affinity for their ligands, ephrins (Eph receptor interacting protein). EPHA2 is also referred to an epithelial cell kinase for its expression in the majority of epithelial cells.
The EphA2 contains an extracellular conserved N-terminal ligand-binding domain followed by a cysteine rich domain with an EGF-like motif and two fibronectin type-III repeats. The extracellular motif is followed by a membrane-spanning region and a cytoplasmic region that encompasses a juxtamembrane region, a tyrosine kinase domain, a sterile alpha motif (SAM), and a post synaptic domain disc large and zona occludens protein (PDZ) binding motif.
Eph--ephrin signaling functions like the classical tyrosine kinase-receptor-mediated cell signaling wherein a cell bearing an Eph receptor upon binding to ephrinA ligand transmits signals downstream known as forward signaling. Eph--ephrin signaling plays essential role in the development of the nervous system and a wide range of functions such as the development of neuronal networks, axon guidance, formation and remodeling of synaptic connections, and nervous system repair. The Eph--ephrin interaction also regulates remodeling of vascular network formation during embryonic development.
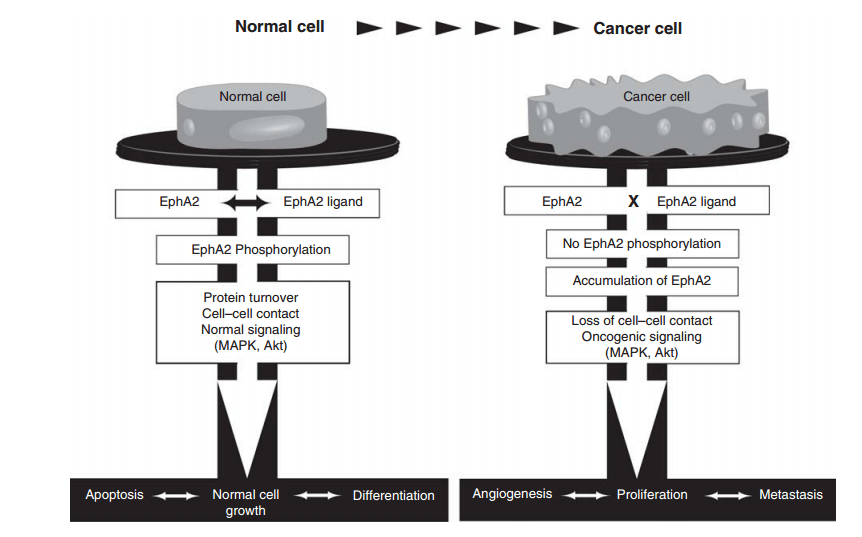
Figure 1. The role of EphA2 in the normal versus the cancer cell
Besides its expression in embryonic and in normal adult tissues, EphA2 is overexpressed in several cancers. In particular, a high level of EphA2 is detected in malignant cancer derived cell lines and advanced forms of cancer. Normally, epha2 becomes phosphorylated and subsequently degraded and this phosphorylated process is important for normal signaling pathway such as MAPK and Akt. In cancer cells, EphA2 fails to efficiently interact with its ligand on adjacent cells, leading to the accumulation of the un-phosphorylated form of EphA2 in the cell. This leads to oncogenic signaling. The increased level of EphA2 expression could be informative in both the prediction of cancer outcomes and in the clinical management of cancer. The differential expression of EphA2 in normal cells compared with cancer cells also signifies its importance as a therapeutic target.
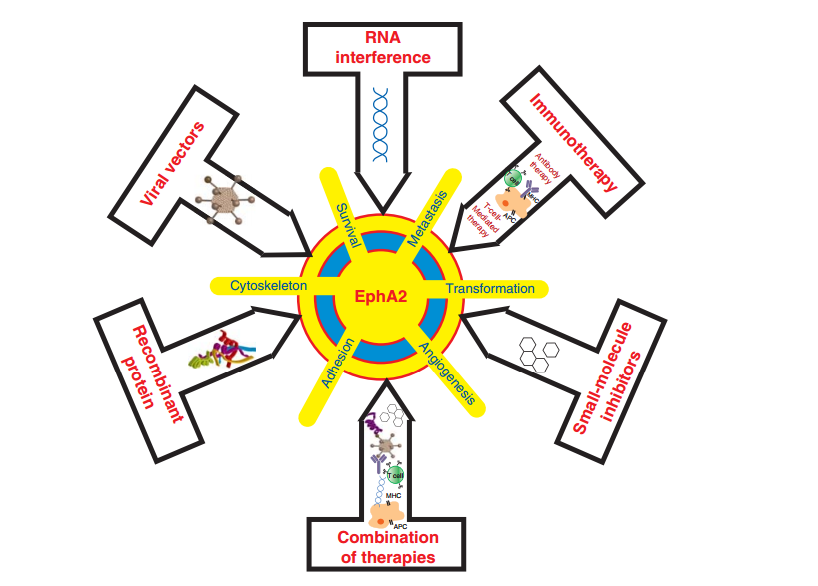
Figure 2. Target epha2 in breast cancer
The EphA2-ephrinA1 signaling axis regulates multiple events that are critical for the malignant transformation of normal cells. EphA2 has been tested as a drug target using multiple approaches such as agonist antibodies, RNA interference, immunotherapy, virus vector-mediated gene transfer, small-molecule inhibitors and nanoparticles. , EphA2 overexpression enhances the resistance of breast cancer cells towards trastuzumab. The inhibition of EphA2 by an anti-EphA2 antibody restored the sensitivity in trastuzumab-resistant cells in in vitro and in orthotopic xenograft models.
Another small-molecule tyrosine kinase inhibitor, dasatinib, has been reported to possess potent inhibitory activity against EphA2. Doxazosins, a novel agonist of EphA2 inhibit Akt and ERK kinase activities in an EphA2-dependent manner. Treatment with doxazosin triggered EphA2 receptor internalization, and suppressed haptotactic and chemotactic migration of prostate cancer, breast cancer, and glioma cells.
References:
Tandon M, Vemula S V, Mittal S K. Emerging strategies for EphA2 receptor targeting for cancer therapeutics[J]. Expert opinion on therapeutic targets, 2011, 15(1): 31-51.
Petty A, Myshkin E, Qin H, et al. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo[J]. PLoS One, 2012, 7(8): e42120.