MemPro™ VEGF receptors
Creative Biostructure provides custom MemPro™ gene-to-structure services for VEGF receptors.
Vascular endothelial growth factor (VEGF) activates three tyrosine kinases, VEGFR-1, VEGFR-2 and VEGFR-3 promoting angiogenic and lymphangiogenic signaling. VEGFs represent a large family of ligands including VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF). Ligand binding promotes receptor dimerization and instigates transmembrane signaling and receptor kinase activation. VEGFRs play essential roles in wound healing and female reproductive cycle, while aberrant angiogenesis and vasculogenesis are observed in a number of pathological conditions such as rheumatoid arthritis, psoriasis, retinal complications and tumor growth. VEGF has been shown to be secreted by tumor cells thereby encouraging new growth and maintenance of a vascular network and culminating in promotion of metastatic spread.
The extracellular receptor domain (ECD) of VEGFR-2 consists of 7 Ig-homology domains. The first 3 domains mediate ligand binding whereas the membrane proximal domains are involved in ligand-induced receptor dimerization. Homotypic receptor interactions in ligand-bound dimers were subsequently identified in Ig-homology domains D4 and D7 using single-particle electron microscopy (EM) and small-angle X-ray scattering (SAXS).
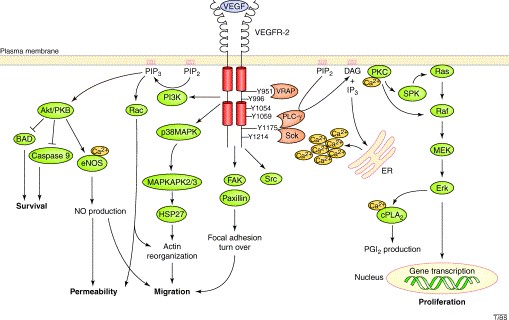
Figure 1. VEGFR activated pathway
Cancer angiogenesis is a fundamental process for the tumor growth as it ensures oxygen and nutrients supply to proliferating cells through the development of new blood vessels, potentially causing cancer progression and metastasis. VEGF-A signaling through VEGFR-2 is the major angiogenic signaling pathway. The binding of VEGF-A to VEGFR2 induces a cascade of different signaling pathways. The dimerization of the receptor and the following autophosphorylation of the intracellular TK domains lead to the simultaneous activation of PLC-γ-Raf kinase-MEK-MAP kinase and PI3K-AKT pathways, causing cellular proliferation and endothelial-cell survival. Thus, specific inhibition of signal transduction via the VEGF-A / VEGFR-2 system is a promising approach to starve the tumor cells of nutrients and thus impede tumor growth and metastasis.
Ramucirumab is a novel human IgG1 mAb that selectively inhibits the VEGFR2 and blocks the VEGFR2-related signaling and activating pathways. Preclinical models showed that ramucirumab selectively binds to the extracellular domain of human VEGFR2 with half-maximal inhibitory concentration of 0.8 to 1.0 nM and it has an 8-fold higher affinity for VEGFR2 when compared with its natural ligands. In 2014, FDA approved single agent ramucirumab for the treatment of patients with advanced or metastatic, gastric or GEJ adenocarcinoma with disease progression on or after prior treatment with fluoropyrimidine- or platinum-containing chemotherapy.
Many small molecules may also inhibit VEGFR2, even if the inhibition is not specific. Sorafenib, has been approved for the first-line treatment of renal cell carcinoma (RCC) and hepatocarcinoma (HCC). sunitinib, has also been approved for RCC and gastrointestinal stromal tumors (GIST), and pazopanib, has recently approved for RCC and soft tissue sarcoma. Moreover, apatinib is a small molecule that may specifically inhibit VEGFR2. Apatinib is a novel, potent VEGFR inhibitor with an intriguing biologic rationale that is able to circumvent cancer cell resistance to other antineoplastic agent. This successful drug is currently being tested in patients with breast or lung cancers.
Reference:
Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors[J]. Annals of translational medicine, 2014, 2(12).
Bold G, Schnell C, Furet P, et al. A Novel Potent Oral Series of VEGFR2 Inhibitors Abrogate Tumor Growth by Inhibiting Angiogenesis[J]. Journal of medicinal chemistry, 2015, 59(1): 132-146.
