Mempro™ GPI anchored protein
Glycosylphosphatidylinositol (GPI) is a lipid anchor for many cell-surface proteins. The GPI anchor represents a post-translational modification of proteins with a glycolipid and is used ubiquitously in eukaryotes and most likely in some Archaea, but not in Eubacteria. GPI-anchored proteins are the major form of cell-surface proteins in protozoa. There are at least 150 GPI-anchored proteins in humans, and they may have a variety of functions serving as receptors, adhesion molecules, enzymes, transcytotic receptors and transporters, and protease inhibitors.
Structure of the GPI Anchor
Detailed analysis of the structure of GPI anchors from a variety of organisms has revealed a common core motif. Through a phosphoethanolamine bridge, the a-carboxyl group at the C-terminus of the mature protein is linked to a highly conserved core glycan. In turn, a lipid moiety is linked by a phosphodiester bridge to the inositol ring. The lipid moiety attaches to inositol and the core glycan can be extensively modified by the addition of both ethanolamine and sugar side chains. The types of GPI anchor modification can be protein, tissue or species specific. A second lipid group, such as palmitic acid, can also be attached to the inositol ring and this modification leaves the GPI anchor resistant to cleavage by PI-PLC. Another common modification is the remodelling of the fatty acids within the GPI anchor, which must take place to allow the protein to associate with lipid rafts.
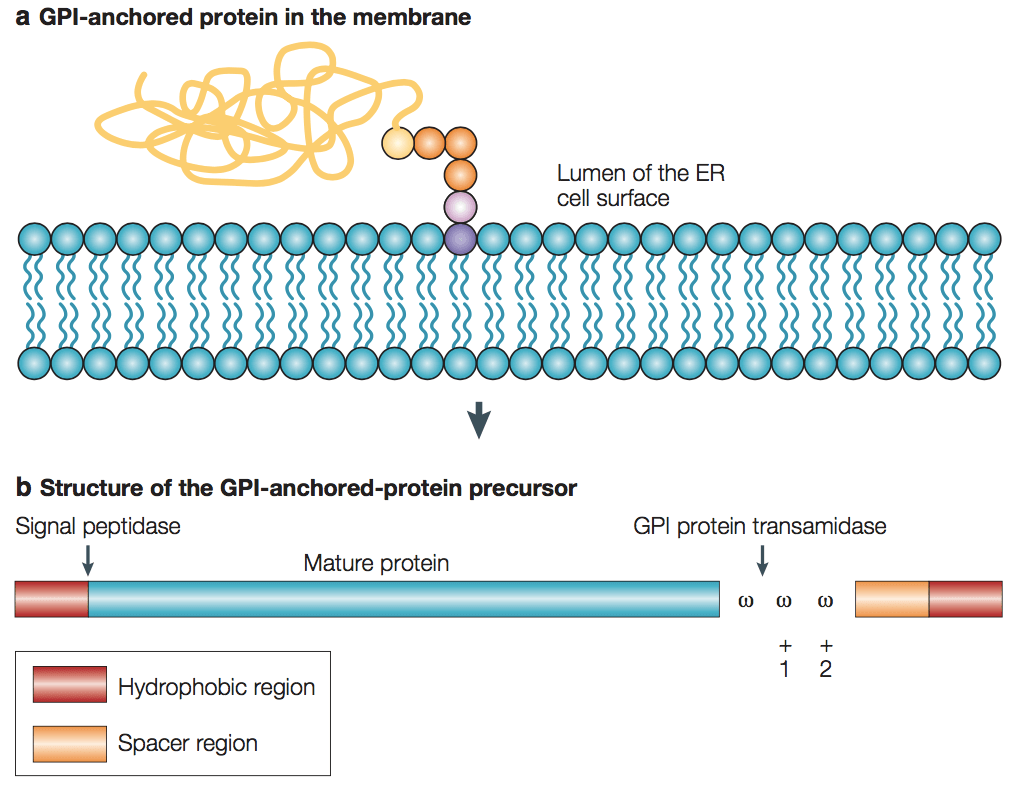 Figure 1. GPI-anchored-protein precursor and anchor structure.
(Satyajit Mayor and Howard Riezman, 2004)
Figure 1. GPI-anchored-protein precursor and anchor structure.
(Satyajit Mayor and Howard Riezman, 2004)
It is of paramount importance to understand the mechanism and functions which GPI-anchored proteins play roles in deseases, and how their structures are recruited/utilized in lipid-based sorting mechanisms. This will provide a basis to understand a role for ‘functional lipid organization’ or ‘lipid rafts’. The conserved core of the GPI-anchor is probably important for specifying such an organization. So it is of great importance to obtain more structures of the GPI moiety on proteins that are expressed in functional contexts. This will greatly aid the rationalization of their role in sorting and specifying a functional organization. These structures might also help to define potential targeting- signal roles for the GPI moiety.
Creative Biostructure is also expertized in development and production of GPI anchored proteins, from expression in various system to crystallization and structure determination. Please feel free to contact us for a detailed quote.
References:
Taroh Kinoshita et al, Thematic Review Series: Glycosylphosphatidylinositol (GPI) Anchors: Biochemistry and Cell Biology. The Journal of Lipid Research. November 18, 2015, doi:10.1194/jlr.E065417
McConville, M.J., and Ferguson, M.A. (1993). The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 294:305–324.
Maeda, Y., Tashima, Y., Houjou, T., et al. (2007). Fatty acid remodeling of GPI-anchored proteins is required for their raft association. Mol. Biol. Cell 18:1497–1506.
Satyajit Mayor and Howard Riezman, Sorting GPI-anchored proteins. NATURE REVIEWS | MOLECULAR CELL BIOLOGY, VOLUME 5 | FEBRUARY 2004.