Custom MemPro™ Cytochromes P450 (CYPs)
Creative Biostructure has developed custom MemPro™ gene-to-structure services for CYPs. CYPs are a superfamily of hemoprotein enzymes, widely distributed in organisms including animals, fungi, bacterial, virus, etc. They interact with CYP reductase (CPR), the requisite partner, and play vital roles in metabolic processes, such as hydroxylation, sulfoxidation and epoxidation. Based on their different components used to transfer electrons during mono-oxygenation, CYPs can be categorized into microsomal P450 systems, mitochondrial P450 systems, bacterial P450 systems, CYB5R/cyb5/P450 systems, FMN/Fd/P450 systems and P450 only systems.
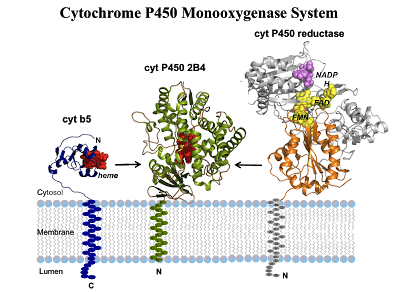
CYPs are versatile biocatalysts. They are heme b containing monooxygenases constituting a superfamily. CYPs have crucial roles in both primary and secondary metabolic pathways. All CYPs share many common properties. Firstly, as shown in the figure, heme is linked to the apoprotein via a conserved cysteine. And the cysteine is the only definitely conserved residue in all CYPs sequences. Mutation of the cysteine will result in the loss of the thiolate ligand which is important in the formation of Fe (IV) species and loss of CYPs function. Secondly, all CYPs except thromboxane synthase and allene oxide synthase are capable of binding and activating atmospheric dioxygen, which play crucial role in the myriad of chemical reactions. Finally, the X-ray crystallographic resolution of many CYPs reveals that they share similar structure and shape. Originally, the P450-redox systems can be divided into two classes. For prokaryotic P450s, class I contains the cytosolic P450–Fdx–FDR electron chain while class II contains the ER (endoplasmic reticulum) membrane bound P450–CPR electron chain. Now a number of new electron chains, which belong neither to class I nor to class II, have been characterized and identified. CYPs catalyze a wide variety of oxidation reactions and they can be engineered by different approaches including random mutagenesis rational design. Properties of some eukaryotic and prokaryotic CPYs have been successfully engineered by means of protein engineering. These methods provide an access to novel components with designed target properties.
Creative Biostructure can provide custom MemPro™ gene-to-structure services for membrane proteins. Please click for more information.
David C. Lamb and Michael R. Waterman. Unusual properties of the cytochrome P450 superfamily. Philos Trans R Soc Lond B Biol Sci. 2013 Jan 6;368(1612):20120434.
Vlada B. Urlacher and Marco Girhard. Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol. 2012 Jan;30(1):26-36.